Two novel missense mutations in the myostatin gene identified in Japanese patients with Duchenne muscular dystrophy
- PMID: 17428346
- PMCID: PMC1855920
- DOI: 10.1186/1471-2350-8-19
Two novel missense mutations in the myostatin gene identified in Japanese patients with Duchenne muscular dystrophy
Abstract
Background: Myostatin is a negative regulator of skeletal muscle growth. Truncating mutations in the myostatin gene have been reported to result in gross muscle hypertrophy. Duchenne muscular dystrophy (DMD), the most common lethal muscle wasting disease, is a result of an absence of muscle dystrophin. Although this disorder causes a rather uniform pattern of muscle wasting, afflicted patients display phenotypic variability. We hypothesized that genetic variation in myostatin is a modifier of the DMD phenotype.
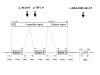
Methods: We analyzed 102 Japanese DMD patients for mutations in the myostatin gene.
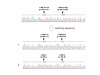
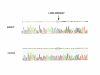
Results: Two polymorphisms that are commonly observed in Western countries, p.55A>T and p.153K>R, were not observed in these Japanese patients. An uncommon polymorphism of p.164E>K was uncovered in four cases; each patient was found to be heterozygous for this polymorphism, which had the highest frequency of the polymorphism observed in the Japanese patients. Remarkably, two patients were found to be heterozygous for one of two novel missense mutations (p.95D>H and p.156L>I). One DMD patient carrying a novel missense mutation of p.95D>H was not phenotypically different from the non-carriers. The other DMD patient was found to carry both a novel mutation (p.156L>I) and a known polymorphism (p.164E>K) in one allele, although his phenotype was not significantly modified. Any nucleotide change creating a target site for micro RNAs was not disclosed in the 3' untranslated region.
Conclusion: Our results indicate that heterozygous missense mutations including two novel mutations did not produce an apparent increase in muscle strength in Japanese DMD cases, even in a patient carrying two missense mutations.
Figures

References
-
- Winnard AV, Klein CJ, Coovert DD, Prior T, Papp A, Snyder P, Bulman DE, Ray PN, McAndrew P, King W, Moxley RT, Mendell JR, Burghes AHM. Characterization of translational frame exception patients in Duchenne/Becker muscular dystrophy. Hum Mol Genet. 1993;2:737–744. doi: 10.1093/hmg/2.6.737. - DOI - PubMed
-
- Prior TW, Bartolo C, Papp AC, Snyder PJ, Sedra MS, Burghes AH, Kissel JT, Luquette MH, Tsao CY, Mendell JR. Dystrophin expression in a Duchenne muscular dystrophy patient with a frame shift deletion. Neurology. 1997;48:486–488. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

