The N-formyl peptide receptors and the anaphylatoxin C5a receptors: an overview
- PMID: 17428601
- PMCID: PMC7115771
- DOI: 10.1016/j.biochi.2007.02.015
The N-formyl peptide receptors and the anaphylatoxin C5a receptors: an overview
Abstract
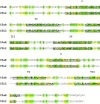
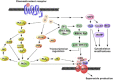
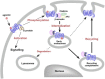
Leukocyte recruitment to sites of inflammation and infection is dependent on the presence of a gradient of locally produced chemotactic factors. This review is focused on current knowledge about the activation and regulation of chemoattractant receptors. Emphasis is placed on the members of the N-formyl peptide receptor family, namely FPR (N-formyl peptide receptor), FPRL1 (FPR like-1) and FPRL2 (FPR like-2), and the complement fragment C5a receptors (C5aR and C5L2). Upon chemoattractant binding, the receptors transduce an activation signal through a G protein-dependent pathway, leading to biochemical responses that contribute to physiological defense against bacterial infection and tissue damage. C5aR, and the members of the FPR family that were previously thought to be restricted to phagocytes proved to have a much broader spectrum of cell expression. In addition to N-formylated peptides, numerous unrelated ligands were recently found to interact with FPR and FPRL1. Novel agonists include both pathogen- and host-derived components, and synthetic peptides. Antagonistic molecules have been identified that exhibit limited receptor specificity. How distinct ligands can both induce different biological responses and produce different modes of receptor activation and unique sets of cellular responses are discussed. Cell responses to chemoattractants are tightly regulated at the level of the receptors. This review describes in detail the regulation of receptor signalling and the multi-step process of receptor inactivation. New concepts, such as receptor oligomerization and receptor clustering, are considered. Although FPR, FPRL1 and C5aR trigger similar biological functions and undergo a rapid chemoattractant-mediated phosphorylation, they appear to be differentially regulated and experience different intracellular fates.
Figures

References
-
- Marasco W.A., Phan S.H., Krutzsch H., Showell H.J., Feltner D.E., Nairn R., Becker E.L., Ward P.A. Purification and identification of formyl-methionyl-leucyl-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J. Biol. Chem. 1984;259:5430–5439. - PubMed
-
- Schiffmann E., Showell H.V., Corcoran B.A., Ward P.A., Smith E., Becker E.L. The isolation and partial characterization of neutrophil chemotactic factors from Escherichia coli. J. Immunol. 1975;114:1831–1837. - PubMed
-
- Ye R., Boulay F. Structure and function of leukocyte chemoattractant receptors. Adv. Pharmacol. 1997;39:221–290. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

