Dynamic decapentaplegic signaling regulates patterning and adhesion in the Drosophila pupal retina
- PMID: 17428827
- PMCID: PMC2957290
- DOI: 10.1242/dev.002972
Dynamic decapentaplegic signaling regulates patterning and adhesion in the Drosophila pupal retina
Abstract
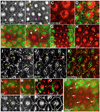
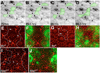
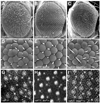
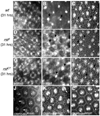
The correct organization of cells within an epithelium is essential for proper tissue and organ morphogenesis. The role of Decapentaplegic/Bone morphogenetic protein (Dpp/BMP) signaling in cellular morphogenesis during epithelial development is poorly understood. In this paper, we used the developing Drosophila pupal retina--looking specifically at the reorganization of glial-like support cells that lie between the retinal ommatidia--to better understand the role of Dpp signaling during epithelial patterning. Our results indicate that Dpp pathway activity is tightly regulated across time in the pupal retina and that epithelial cells in this tissue require Dpp signaling to achieve their correct shape and position within the ommatidial hexagon. These results point to the Dpp pathway as a third component and functional link between two adhesion systems, Hibris-Roughest and DE-cadherin. A balanced interplay between these three systems is essential for epithelial patterning during morphogenesis of the pupal retina. Importantly, we identify a similar functional connection between Dpp activity and DE-cadherin and Rho1 during cell fate determination in the wing, suggesting a broader link between Dpp function and junctional integrity during epithelial development.
Figures



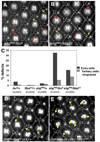
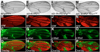
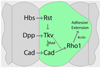
References
-
- Affolter M, Nellen D, Nussbaumer U, Basler K. Multiple requirements for the receptor serine/threonine kinase thick veins reveal novel functions of TGF beta homologs during Drosophila embryogenesis. Development. 1994;120:3105–3117. - PubMed
-
- Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. - PubMed
-
- Bao S, Cagan R. Preferential adhesion mediated by Hibris and Roughest regulates morphogenesis and patterning in the Drosophila eye. Dev. Cell. 2005;8:925–935. - PubMed
-
- Beebe D, Garcia C, Wang X, Rajagopal R, Feldmeier M, Kim JY, Chytil A, Moses H, Ashery-Padan R, Rauchman M. Contributions by members of the TGFbeta superfamily to lens development. Int. J. Dev. Biol. 2004;48:845–856. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

