Epidermal growth factor receptor is not required for human cytomegalovirus entry or signaling
- PMID: 17428848
- PMCID: PMC1900073
- DOI: 10.1128/JVI.00169-07
Epidermal growth factor receptor is not required for human cytomegalovirus entry or signaling
Abstract
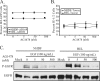
Human cytomegalovirus (HCMV) can bind, fuse, and initiate gene expression in a diverse range of vertebrate cell types. This broad cellular tropism suggests that multiple receptors and/or universally distributed receptors mediate HCMV entry. Our laboratory has recently discovered that certain beta1 and beta3 integrin heterodimers are critical mediators of HCMV entry into permissive fibroblasts (A. L. Feire, H. Koss, and T. Compton, Proc. Natl. Acad. Sci. USA 101:15470-15475, 2004). It has also been reported that epidermal growth factor receptor (EGFR) is necessary for HCMV-mediated signaling and entry (X. Wang, S. M. Huong, M. L. Chiu, N. Raab-Traub, and E. E. Huang, Nature 424:456-461, 2003). Integrins are known to signal synergistically with growth factor receptors, and this coordination was recently reported for EGFR and beta3 integrins in the context of HCMV entry (X. Wang, D. Y. Huang, S. M. Huong, and E. S. Huang, Nat. Med. 11:515-521, 2005). However, EGFR-negative cell lines, such as hematopoietic cells, are known to be infected by HCMV. Therefore, we wished to confirm a role for EGFR in HCMV entry and then examine any interaction between beta1 integrins and EGFR during the entry process. Surprisingly, we were unable to detect any role for EGFR in the process of HCMV entry into fibroblast, epithelial, or endothelial cell lines. Additionally, HCMV did not activate the EGFR kinase in fibroblast cell lines. We first examined HCMV entry into two EGFR-positive or -negative cell lines but observed no increase in entry when EGFR was expressed to high levels. Physically blocking EGFR with a neutralizing antibody in fibroblast, epithelial, or endothelial cell lines or blocking EGFR kinase signaling with a chemical inhibitor in fibroblast cells did not inhibit virus entry. Lastly, we were unable to detect phosphorylation of EGFR in fibroblasts cells in response to HCMV stimulation. Our findings demonstrate that EGFR does not play a significant role in HCMV entry or signaling. These results suggest that specific integrin heterodimers either act alone as the primary entry receptors or interact in conjunction with an additional receptor(s), other than EGFR, to facilitate virus entry.
Figures




References
-
- Adam, E., J. L. Melnick, J. L. Probtsfield, B. L. Petrie, J. Burek, K. R. Bailey, C. H. McCollum, and M. E. DeBakey. 1987. High levels of cytomegalovirus antibody in patients requiring vascular surgery for atherosclerosis. Lancet 2:291-293. - PubMed
-
- Alford, C. A., and W. J. Britt. 1993. Cytomegalovirus, p. 227-255. In B. Roizman, R. J. Whitley, and C. Lopez (ed.), The human herpesviruses. Raven Press, New York, NY.
-
- Compton, T., R. R. Nepomuceno, and D. M. Nowlin. 1992. Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology 191:387-395. - PubMed
-
- Compton, T., D. M. Nowlin, and N. R. Cooper. 1993. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology 193:834-841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

