Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors
- PMID: 17428852
- PMCID: PMC1900096
- DOI: 10.1128/JVI.02497-06
Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors
Abstract
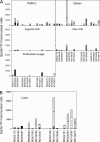
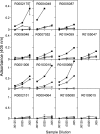
In this study we compared a prime-boost regimen with two serologically distinct replication-defective adenovirus (Ad) vectors derived from chimpanzee serotypes C68 and C1 expressing Gag, Pol, gp140, and Nef of human immunodeficiency virus type 1 with a regimen in which replication-defective Ad vectors of the human serotype 5 (AdHu5) were given twice. Experiments were conducted in rhesus macaques that had or had not been preexposed to antigens of AdHu5. There was no significant difference in T-cell responses tested from peripheral blood of the different groups, although responses were overall highest in nonpreexposed animals immunized with the chimpanzee Ad vectors. Preexisting immunity to AdHu5 completely inhibited induction of transgene product-specific antibodies by the AdHu5 vectors without affecting antibody responses to the chimpanzee vectors. Upon euthanasia, T-cell responses were tested from a number of tissues. Preexisting immunity to AdHu5, commonly found in humans, changed the homing pattern of vaccine-induced T cells. In AdHu5-preexposed animals vaccinated with the chimpanzee Ad vectors, frequencies of transgene-specific T cells were higher in spleens than in blood, and in most preexposed animals vaccinated either with AdHu5 vectors or chimpanzee adenovirus vectors, frequencies of such T cells were exceptionally high in livers. The latter results indicate that analysis of T-cell responses solely from blood mononuclear cells of vaccine recipients may not suffice to compare the potencies of different vaccine regimens.
Figures


References
-
- Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Caulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. - PMC - PubMed
-
- Cohen, P. 2006. Immunity's yin and yang. A successful vaccine must first avoid being eliminated by pre-existing immunity before it can promote a protective immune response. IAVI Rep. 10:1-5. - PubMed
-
- Crystal, R. G. 1992. Gene therapy strategies for pulmonary disease. Am. J. Med. 92:44S-52S. - PubMed
-
- Durier, C., O. Launay, V. Meiffredy, Y. Saidi, D. Salmon, Y. Levy, J. G. Guillet, G. Pialoux, and J. P. Aboulker. 2006. Clinical safety of HIV lipopeptides used as vaccines in healthy volunteers and HIV-infected adults. AIDS 20:1039-1049. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

