HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype
- PMID: 17428922
- PMCID: PMC1851664
- DOI: 10.1073/pnas.0611244104
HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype
Abstract
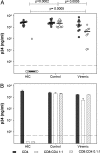
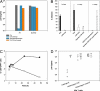
Some rare HIV-1-infected individuals, referred to as HIV controllers (HIC), have persistently undetectable plasma viral load in the absence of therapy. This control of HIV-1 replication has been associated with a strong, multifunctional specific CD8(+) T cell response. However, no direct link between this immune response and the control of viremia has so far been provided. We investigated parameters of specific CD8(+) T cell response and in vitro susceptibility to HIV-1 infection in 11 HIC. We found high frequencies of HIV-specific CD8(+) T cells. Interestingly, these cells expressed the activation marker HLA-DR but not CD38. This unique phenotype differentiates HIV-specific CD8(+) T cells from HIC and noncontroller subjects and likely reflects a high potential to expand upon exposure to antigen and a capacity to exert effector functions. Accordingly, although CD4(+) T cells from HIC were fully susceptible to HIV-1 superinfection, their CD8(+) T cells effectively suppressed HIV-1 infection. Remarkably, this potent anti-HIV activity was observed without prior stimulation of CD8(+) T cells. This activity was not mediated by secreted inhibitory factors but was due to the elimination of infected CD4(+) T cells and was observed only with autologous CD4(+) T cells, indicating an HLA-restricted cytotoxic mechanism. This constitutive antiviral capacity of CD8(+) T cells could account for the control of viral replication in HIC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
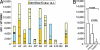

References
-
- Cao Y, Qin L, Zhang L, Safrit J, Ho DD. N Engl J Med. 1995;332:201–208. - PubMed
-
- Harrer T, Harrer E, Kalams SA, Elbeik T, Staprans SI, Feinberg MB, Cao Y, Ho DD, Yilma T, Caliendo AM, et al. AIDS Res Hum Retroviruses. 1996;12:585–592. - PubMed
-
- Kloosterboer N, Groeneveld PH, Jansen CA, van der Vorst TJ, Koning F, Winkel CN, Duits AJ, Miedema F, van Baarle D, van Rij RP, et al. Virology. 2005;339:70–80. - PubMed
-
- Lambotte O, Boufassa F, Madec Y, Nguyen A, Goujard C, Meyer L, Rouzioux C, Venet A, Delfraissy JF. Clin Infect Dis. 2005;41:1053–1056. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

