Anti-GM1 antibodies cause complement-mediated disruption of sodium channel clusters in peripheral motor nerve fibers
- PMID: 17428969
- PMCID: PMC6672537
- DOI: 10.1523/JNEUROSCI.4401-06.2007
Anti-GM1 antibodies cause complement-mediated disruption of sodium channel clusters in peripheral motor nerve fibers
Abstract
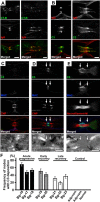
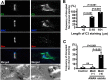
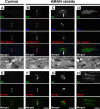
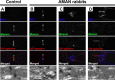
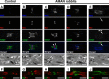
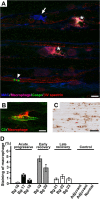
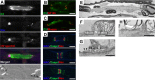
Voltage-gated Na+ (Na(v)) channels are highly concentrated at nodes of Ranvier in myelinated axons and facilitate rapid action potential conduction. Autoantibodies to gangliosides such as GM1 have been proposed to disrupt nodal Nav channels and lead to Guillain-Barré syndrome, an autoimmune neuropathy characterized by acute limb weakness. To test this hypothesis, we examined the molecular organization of nodes in a disease model caused by immunization with gangliosides. At the acute phase with progressing limb weakness, Na(v) channel clusters were disrupted or disappeared at abnormally lengthened nodes concomitant with deposition of IgG and complement products. Paranodal axoglial junctions, the nodal cytoskeleton, and Schwann cell microvilli, all of which stabilize Na(v) channel clusters, were also disrupted. The nodal molecules disappeared in lesions with complement deposition but no localization of macrophages. During recovery, complement deposition at nodes decreased, and Na(v) channels redistributed on both sides of affected nodes. These results suggest that Na(v) channel alterations occur as a consequence of complement-mediated disruption of interactions between axons and Schwann cells. Our findings support the idea that acute motor axonal neuropathy is a disease that specifically disrupts the nodes of Ranvier.
Figures

References
-
- Arroyo EJ, Sirkowski EE, Chitale R, Scherer SS. Acute demyelination disrupts the molecular organization of peripheral nervous system nodes. J Comp Neurol. 2004;479:424–434. - PubMed
-
- Berghs S, Aggujaro D, Dirkx R, Jr, Maksimova E, Stabach P, Hermel JM, Zhang JP, Philbrick W, Slepnev V, Ort T, Solimena M. βIV spectrin, a new spectrin localized at axon initial segments and nodes of Ranvier in the central and peripheral nervous system. J Cell Biol. 2000;151:985–1001. - PMC - PubMed
-
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St. Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. - PubMed
-
- Caporale CM, Capasso M, Luciani M, Prencipe V, Creati B, Gandolfi P, De Angelis MV, Di Muzio A, Caporale V, Uncini A. Experimental axonopathy induced by immunization with Campylobacter jejuni lipopolysaccharide from a patient with Guillain-Barré syndrome. J Neuroimmunol. 2006;174:12–20. - PubMed
-
- Comín R, Yuki N, Lopez PHH, Nores GA. High affinity of anti-GM1 antibodies is associated with disease onset in experimental neuropathy. J Neurosci Res. 2006;84:1085–1090. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
