Actin filaments mediate mechanical gating during osmosensory transduction in rat supraoptic nucleus neurons
- PMID: 17428977
- PMCID: PMC6672547
- DOI: 10.1523/JNEUROSCI.3278-06.2007
Actin filaments mediate mechanical gating during osmosensory transduction in rat supraoptic nucleus neurons
Abstract
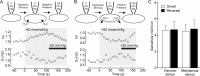
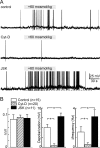
Osmosensory transduction is a bidirectional process displayed by neurons involved in the control of thirst and antidiuretic hormone release, and is therefore crucial for body fluid homeostasis. Although this mechanism is known to involve the activation of nonselective cation channels during hypertonicity-evoked shrinking, and the inhibition of these channels during hypotonicity-evoked swelling, the basis for this regulation is unknown. Here, we investigated this process using whole-cell patch-clamp recordings from neurons acutely isolated from the supraoptic nucleus of adult rats. The mechanosensitivity index, defined as the ratio of conductance change to normalized volume change, was quantitatively equivalent whether cell volume was increased or decreased by changes in extracellular fluid osmolality, or by changes in pipette pressure. Moreover, responses induced by hyperosmotic or hypo-osmotic media could be reversed by increasing or decreasing pipette pressure, respectively. The mechanosensitivity index was significantly reduced in neurons treated with cytochalasin-D, a compound that promotes the depolymerization of actin filaments. Conversely, cells treated with jasplakinolide, a compound that promotes actin polymerization, showed a significant increase in mechanosensitivity index. Finally, the depolarizing and excitatory effects of hypertonic stimuli were significantly enhanced by jasplakinolide and reduced by cytochalasin-D. We conclude that osmosensory transduction in these neurons is a reversible mechanical process that depends on an intact actin cytoskeleton, and the sensitivity of the transducer appears to vary in proportion with the density of actin filaments.
Figures


Similar articles
-
Mechanical basis of osmosensory transduction in magnocellular neurosecretory neurones of the rat supraoptic nucleus.J Neuroendocrinol. 2015 Jun;27(6):507-15. doi: 10.1111/jne.12270. J Neuroendocrinol. 2015. PMID: 25712904 Review.
-
Amplification of transducer gain by angiotensin II-mediated enhancement of cortical actin density in osmosensory neurons.J Neurosci. 2008 Sep 17;28(38):9536-44. doi: 10.1523/JNEUROSCI.1495-08.2008. J Neurosci. 2008. PMID: 18799685 Free PMC article.
-
Coincident detection of CSF Na+ and osmotic pressure in osmoregulatory neurons of the supraoptic nucleus.Neuron. 1999 Oct;24(2):453-60. doi: 10.1016/s0896-6273(00)80858-2. Neuron. 1999. PMID: 10571238
-
Stretch-inactivated cation channels: cellular targets for modulation of osmosensitivity in supraoptic neurons.Prog Brain Res. 2002;139:85-94. doi: 10.1016/s0079-6123(02)39009-5. Prog Brain Res. 2002. PMID: 12436928 Review.
-
Gadolinium uncouples mechanical detection and osmoreceptor potential in supraoptic neurons.Neuron. 1996 Jan;16(1):175-81. doi: 10.1016/s0896-6273(00)80034-3. Neuron. 1996. PMID: 8562082
Cited by
-
Submucosal enteric neurons of the cavine distal colon are sensitive to hypoosmolar stimuli.J Physiol. 2020 Dec;598(23):5317-5332. doi: 10.1113/JP280309. Epub 2020 Sep 16. J Physiol. 2020. PMID: 32880976 Free PMC article.
-
ATP stimulates rat hypothalamic sympathetic neurons by enhancing AMPA receptor-mediated currents.J Neurophysiol. 2015 Jul;114(1):159-69. doi: 10.1152/jn.01011.2014. Epub 2015 Apr 22. J Neurophysiol. 2015. PMID: 25904713 Free PMC article.
-
Role of Vasopressin in Rat Models of Salt-Dependent Hypertension.Curr Hypertens Rep. 2017 May;19(5):42. doi: 10.1007/s11906-017-0741-2. Curr Hypertens Rep. 2017. PMID: 28451854 Review.
-
Advances in the neurophysiology of magnocellular neuroendocrine cells.J Neuroendocrinol. 2020 Apr;32(4):e12826. doi: 10.1111/jne.12826. Epub 2020 Feb 5. J Neuroendocrinol. 2020. PMID: 31917875 Free PMC article. Review.
-
Hypertonicity sensing in organum vasculosum lamina terminalis neurons: a mechanical process involving TRPV1 but not TRPV4.J Neurosci. 2011 Oct 12;31(41):14669-76. doi: 10.1523/JNEUROSCI.1420-11.2011. J Neurosci. 2011. PMID: 21994383 Free PMC article.
References
-
- Bourque CW, Oliet SH, Richard D. Osmoreceptors, osmoreception, and osmoregulation. Front Neuroendocrinol. 1994;15:231–274. - PubMed
-
- Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem. 1994;269:14869–14871. - PubMed
-
- Cannon CL, Basavappa S, Strange K. Intracellular ionic strength regulates the volume sensitivity of a swelling-activated anion channel. Am J Physiol. 1998;275:C416–C422. - PubMed
-
- Cicchetti G, Allen PG, Glogauer M. Chemotactic signaling pathways in neutrophils: from receptor to actin assembly. Crit Rev Oral Biol Med. 2002;13:220–228. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
