Muscarinic control of long-range GABAergic inhibition within the rhinal cortices
- PMID: 17428984
- PMCID: PMC6672538
- DOI: 10.1523/JNEUROSCI.0068-07.2007
Muscarinic control of long-range GABAergic inhibition within the rhinal cortices
Abstract
The perirhinal cortex plays a critical role in memory formation, in part because it forms reciprocal connections with the neocortex and entorhinal cortex and is thus in a position to integrate and transfer higher-order information to and from the hippocampus. However, for reasons that remain unclear, perirhinal transfer of neocortical inputs to the entorhinal cortex occurs with a low probability. Using patch recordings in vitro and tract-tracing combined with GAD-67 immunohistochemistry, we show that the perirhinal cortex contains GABAergic neurons with long-range projections to superficial entorhinal cells. This finding challenges the traditional model of cortical inhibition in which all trans-areal inhibition is thought to be disynaptic because the axons of GABAergic interneurons are assumed to be confined within the area in which their somata are located. Moreover, consistent with recent studies indicating that the formation of perirhinal-dependent memories requires activation of muscarinic receptors, long-range IPSPs were presynaptically inhibited by M2 receptor activation. Overall, these results suggest that long-range feedforward inhibition regulates perirhinal transfer of neocortical inputs to the entorhinal cortex, but that cholinergic inputs can presynaptically adjust the impact of this control mechanism as a function of environmental contingencies.
Figures
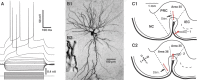


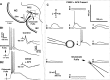

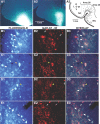

References
-
- Abe H, Ishida Y, Iwasaki T. Perirhinal N-methyl-d-aspartate and muscarinic systems participate in object recognition in rats. Neurosci Lett. 2004;356:191–194. - PubMed
-
- Aggleton JP, Hunt PR, Rawlins JN. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behav Brain Res. 1986;19:133–146. - PubMed
-
- Alonso A. Electrophysiology of neurones in the perirhinal and entorhinal cortices and neuromodulatory changes in firing patterns. In: Witter MP, Wouterlood F, editors. The parahippocampal region. Oxford: Oxford UP; 2002. pp. 89–105.
-
- Behrends JC, ten Bruggencate G. Cholinergic modulation of synaptic inhibition in the guinea pig hippocampus in vitro: excitation of GABAergic interneurons and inhibition of GABA-release. J Neurophysiol. 1993;69:626–629. - PubMed
-
- Bekkers JM, Stevens CF. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990;346:724–729. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
