Inositol trisphosphate receptor Ca2+ release channels
- PMID: 17429043
- PMCID: PMC2901638
- DOI: 10.1152/physrev.00035.2006
Inositol trisphosphate receptor Ca2+ release channels
Abstract
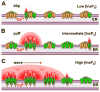
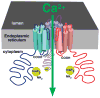
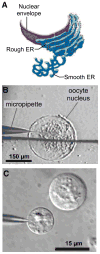
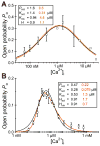
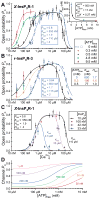
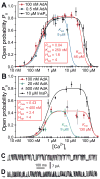
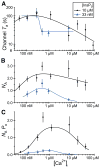
The inositol 1,4,5-trisphosphate (InsP3) receptors (InsP3Rs) are a family of Ca2+ release channels localized predominately in the endoplasmic reticulum of all cell types. They function to release Ca2+ into the cytoplasm in response to InsP3 produced by diverse stimuli, generating complex local and global Ca2+ signals that regulate numerous cell physiological processes ranging from gene transcription to secretion to learning and memory. The InsP3R is a calcium-selective cation channel whose gating is regulated not only by InsP3, but by other ligands as well, in particular cytoplasmic Ca2+. Over the last decade, detailed quantitative studies of InsP3R channel function and its regulation by ligands and interacting proteins have provided new insights into a remarkable richness of channel regulation and of the structural aspects that underlie signal transduction and permeation. Here, we focus on these developments and review and synthesize the literature regarding the structure and single-channel properties of the InsP3R.
Figures






References
-
- Abe H, Shuto S, Matsuda A. Synthesis of the C-glycosidic analog of adenophostin A and its uracil congener as potential IP3 receptor ligands. Stereoselective construction of the C-glycosidic structure by a temporary silicon-tethered radical coupling reaction. J Org Chem. 2000;65:4315–4325. - PubMed
-
- Abraham RT, Wiederrecht GJ. Immunopharmacology of rapamycin. Annu Rev Immunol. 1996;14:483–510. - PubMed
-
- Acharya JK, Jalink K, Hardy RW, Hartenstein V, Zuker CS. InsP3 receptor is essential for growth and differentiation but not for vision in Drosophila. Neuron. 1997;18:881–887. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

