Cell polarity development and protein trafficking in hepatocytes lacking E-cadherin/beta-catenin-based adherens junctions
- PMID: 17429067
- PMCID: PMC1877101
- DOI: 10.1091/mbc.e06-11-1040
Cell polarity development and protein trafficking in hepatocytes lacking E-cadherin/beta-catenin-based adherens junctions
Abstract
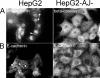
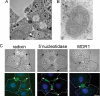
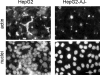
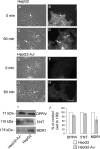
Using a mutant hepatocyte cell line in which E-cadherin and beta-catenin are completely depleted from the cell surface, and, consequently, fail to form adherens junctions, we have investigated adherens junction requirement for apical-basolateral polarity development and polarized membrane trafficking. It is shown that these hepatocytes retain the capacity to form functional tight junctions, develop full apical-basolateral cell polarity, and assemble a subapical cortical F-actin network, although with a noted delay and a defect in subsequent apical lumen remodeling. Interestingly, whereas hepatocytes typically target the plasma membrane protein dipeptidyl peptidase IV first to the basolateral surface, followed by its transcytosis to the apical domain, hepatocytes lacking E-cadherin-based adherens junctions target dipeptidyl peptidase IV directly to the apical surface. Basolateral surface-directed transport of other proteins or lipids tested was not visibly affected in hepatocytes lacking E-cadherin-based adherens junctions. Together, our data show that E-cadherin/beta-catenin-based adherens junctions are dispensable for tight junction formation and apical lumen biogenesis but not for apical lumen remodeling. In addition, we suggest a possible requirement for E-cadherin/beta-catenin-based adherens junctions with regard to the indirect apical trafficking of specific proteins in hepatocytes.
Figures




References
-
- Aït Slimane T., Lenoir C., Bello V., Delaunay J. L., Goding J. W., Chwetzoff S., Maurice M., Fransen J. A., Trugnan G. The cytoplasmic/transmembrane domain of dipeptidyl peptidase IV, a type II glycoprotein, contains an apical targeting signal that does not specifically interact with lipid rafts. Exp. Cell Res. 2001;270:45–55. - PubMed
-
- Baas A. F., Kuipers J., van der Wel N. N., Batlle E., Koerten H. K., Peters P. J., Clevers H. C. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004;116:457–466. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

