Direct observation of regulated ribonucleoprotein transport across the nurse cell/oocyte boundary
- PMID: 17429069
- PMCID: PMC1877097
- DOI: 10.1091/mbc.e06-10-0959
Direct observation of regulated ribonucleoprotein transport across the nurse cell/oocyte boundary
Abstract
In Drosophila, the asymmetric localization of specific mRNAs to discrete regions within the developing oocyte determines the embryonic axes. The microtubule motors dynein and kinesin are required for the proper localization of the determinant ribonucleoprotein (RNP) complexes, but the mechanisms that account for RNP transport to and within the oocyte are not well understood. In this work, we focus on the transport of RNA complexes containing bicoid (bcd), an anterior determinant. We show in live egg chambers that, within the nurse cell compartment, dynein actively transports green fluorescent protein-tagged Exuperantia, a cofactor required for bcd RNP localization. Surprisingly, the loss of kinesin I activity elevates RNP motility in nurse cells, whereas disruption of dynein activity inhibits RNP transport. Once RNPs are transferred through the ring canal to the oocyte, they no longer display rapid, linear movements, but they are distributed by cytoplasmic streaming and gradually disassemble. By contrast, bcd mRNA injected into oocytes assembles de novo into RNP particles that exhibit rapid, dynein-dependent transport. We speculate that after delivery to the oocyte, RNP complexes may disassemble and be remodeled with appropriate accessory factors to ensure proper localization.
Figures
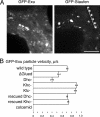
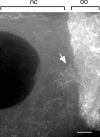
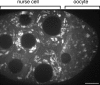

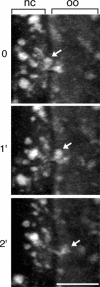
References
-
- Brand A. H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

