Loss of cell adhesion causes hydrocephalus in nonmuscle myosin II-B-ablated and mutated mice
- PMID: 17429076
- PMCID: PMC1877086
- DOI: 10.1091/mbc.e07-01-0073
Loss of cell adhesion causes hydrocephalus in nonmuscle myosin II-B-ablated and mutated mice
Abstract
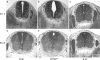
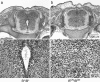
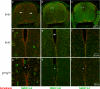
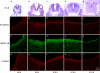
Ablation of nonmuscle myosin (NM) II-B in mice during embryonic development leads to marked enlargement of the cerebral ventricles and destruction of brain tissue, due to hydrocephalus. We have identified a transient mesh-like structure present at the apical border of cells lining the spinal canal of mice during development. This structure, which only contains the II-B isoform of NM, also contains beta-catenin and N-cadherin, consistent with a role in cell adhesion. Ablation of NM II-B or replacement of NM II-B with decreased amounts of a mutant (R709C), motor-impaired NM II-B in mice results in collapse of the mesh-like structure and loss of cell adhesion. This permits the underlying neuroepithelial cells to invade the spinal canal and obstruct cerebral spinal fluid flow. These defects in the CNS of NM II-B-ablated mice seem to be the cause of hydrocephalus. Interestingly, the mesh-like structure and patency of the spinal canal can be restored by increasing expression of the motor-impaired NM II-B, which also rescues hydrocephalus. However, the mutant isoform cannot completely rescue neuronal cell migration. These studies show that the scaffolding properties of NM II-B play an important role in cell adhesion, thereby preventing hydrocephalus during mouse brain development.
Figures





Similar articles
-
Ablation of nonmuscle myosin II-B and II-C reveals a role for nonmuscle myosin II in cardiac myocyte karyokinesis.Mol Biol Cell. 2010 Nov 15;21(22):3952-62. doi: 10.1091/mbc.E10-04-0293. Epub 2010 Sep 22. Mol Biol Cell. 2010. PMID: 20861308 Free PMC article.
-
Nonmuscle myosin II isoform and domain specificity during early mouse development.Proc Natl Acad Sci U S A. 2010 Aug 17;107(33):14645-50. doi: 10.1073/pnas.1004023107. Epub 2010 Aug 2. Proc Natl Acad Sci U S A. 2010. PMID: 20679233 Free PMC article.
-
Replacement of nonmuscle myosin II-B with II-A rescues brain but not cardiac defects in mice.J Biol Chem. 2007 Jul 27;282(30):22102-11. doi: 10.1074/jbc.M702731200. Epub 2007 May 22. J Biol Chem. 2007. PMID: 17519229
-
Distinct and redundant roles of the non-muscle myosin II isoforms and functional domains.Biochem Soc Trans. 2011 Oct;39(5):1131-5. doi: 10.1042/BST0391131. Biochem Soc Trans. 2011. PMID: 21936777 Free PMC article. Review.
-
Mammalian nonmuscle myosin II comes in three flavors.Biochem Biophys Res Commun. 2018 Nov 25;506(2):394-402. doi: 10.1016/j.bbrc.2018.03.103. Epub 2018 Mar 17. Biochem Biophys Res Commun. 2018. PMID: 29550471 Free PMC article. Review.
Cited by
-
PTK7 regulates myosin II activity to orient planar polarity in the mammalian auditory epithelium.Curr Biol. 2012 Jun 5;22(11):956-66. doi: 10.1016/j.cub.2012.03.068. Epub 2012 May 3. Curr Biol. 2012. PMID: 22560610 Free PMC article.
-
Ablation of nonmuscle myosin II-B and II-C reveals a role for nonmuscle myosin II in cardiac myocyte karyokinesis.Mol Biol Cell. 2010 Nov 15;21(22):3952-62. doi: 10.1091/mbc.E10-04-0293. Epub 2010 Sep 22. Mol Biol Cell. 2010. PMID: 20861308 Free PMC article.
-
Tissue organization by cadherin adhesion molecules: dynamic molecular and cellular mechanisms of morphogenetic regulation.Physiol Rev. 2011 Apr;91(2):691-731. doi: 10.1152/physrev.00004.2010. Physiol Rev. 2011. PMID: 21527735 Free PMC article. Review.
-
A novel interaction of CLN3 with nonmuscle myosin-IIB and defects in cell motility of Cln3(-/-) cells.Exp Cell Res. 2011 Jan 1;317(1):51-69. doi: 10.1016/j.yexcr.2010.09.007. Epub 2010 Sep 17. Exp Cell Res. 2011. PMID: 20850431 Free PMC article.
-
Myosin II regulates extension, growth and patterning in the mammalian cochlear duct.Development. 2009 Jun;136(12):1977-86. doi: 10.1242/dev.030718. Epub 2009 May 13. Development. 2009. PMID: 19439495 Free PMC article.
References
-
- Bao J., Jana S. S., Adelstein R. S. Vertebrate nonmuscle myosin II isoforms rescue small interfering RNA-induced defects in COS-7 cell cytokinesis. J. Biol. Chem. 2005;280:19594–19599. - PubMed
-
- Buxton D. B., Golomb E., Adelstein R. S. Induction of nonmuscle myosin heavy chain II-C by butyrate in RAW 264.7 mouse macrophages. J. Biol. Chem. 2003;278:15449–15455. - PubMed
-
- Conti M. A., Even-Ram S., Liu C., Yamada K. M., Adelstein R. S. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J. Biol. Chem. 2004;279:41263–41266. - PubMed
-
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987;236:1086–1091. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

