Adaptive optics-optical coherence tomography: optimizing visualization of microscopic retinal structures in three dimensions
- PMID: 17429483
- PMCID: PMC2583222
- DOI: 10.1364/josaa.24.001373
Adaptive optics-optical coherence tomography: optimizing visualization of microscopic retinal structures in three dimensions
Abstract
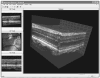
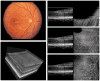
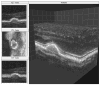
Adaptive optics-optical coherence tomography (AO-OCT) permits improved imaging of microscopic retinal structures by combining the high lateral resolution of AO with the high axial resolution of OCT, resulting in the narrowest three-dimensional (3D) point-spread function (PSF) of all in vivo retinal imaging techniques. Owing to the high volumetric resolution of AO-OCT systems, it is now possible, for the first time, to acquire images of 3D cellular structures in the living retina. Thus, with AO-OCT, those retinal structures that are not visible with AO or OCT alone (e.g., bundles of retinal nerve fiber layers, 3D mosaic of photoreceptors, 3D structure of microvasculature, and detailed structure of retinal disruptions) can be visualized. Our current AO-OCT instrumentation uses spectrometer-based Fourier-domain OCT technology and two-deformable-mirror-based AO wavefront correction. We describe image processing methods that help to remove motion artifacts observed in volumetric data, followed by innovative data visualization techniques [including two-dimensional (2D) and 3D representations]. Finally, examples of microscopic retinal structures that are acquired with the University of California Davis AO-OCT system are presented.
Figures




References
-
- Fercher AF, Hitzenberger CK, Kamp G, Elzaiat Y. Measurement of intraocular distances by backscattering spectral interferometry. Opt Commun. 1995;117:43–48.
-
- Häusler G, Lindner MW. Coherence radar and spectral radar—new tools for dermatological diagnosis. J Biomed Opt. 1998;7:21–31. - PubMed
-
- Wojtkowski M, Leitgeb R, Kowalczyk A, Bajraszewski T, Fercher AF. In vivo human retinal imaging by Fourier domain optical coherence tomography. J Biomed Opt. 2002;7:457–463. - PubMed
-
- Wojtkowski M, Bajraszewski T, Targowski P, Kowalczyk A. Real time in vivo imaging by high-speed spectral optical coherence tomography. Opt Lett. 2003;28:1745–1747. - PubMed
-
- Nassif NA, Cense B, Park BH, Pierce MC, Yun SH, Bouma BE, Tearney GJ, Chen TC, de Boer JF. In vivo high-resolution video-rate spectral-domain optical coherence tomography of the human retina and optic nerve. Opt Express. 2004;12:367–376. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

