Ancient papillomavirus-host co-speciation in Felidae
- PMID: 17430578
- PMCID: PMC1896010
- DOI: 10.1186/gb-2007-8-4-r57
Ancient papillomavirus-host co-speciation in Felidae
Abstract
Background: Estimating evolutionary rates for slowly evolving viruses such as papillomaviruses (PVs) is not possible using fossil calibrations directly or sequences sampled over a time-scale of decades. An ability to correlate their divergence with a host species, however, can provide a means to estimate evolutionary rates for these viruses accurately. To determine whether such an approach is feasible, we sequenced complete feline PV genomes, previously available only for the domestic cat (Felis domesticus, FdPV1), from four additional, globally distributed feline species: Lynx rufus PV type 1, Puma concolor PV type 1, Panthera leo persica PV type 1, and Uncia uncia PV type 1.
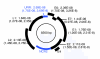
Results: The feline PVs all belong to the Lambdapapillomavirus genus, and contain an unusual second noncoding region between the early and late protein region, which is only present in members of this genus. Our maximum likelihood and Bayesian phylogenetic analyses demonstrate that the evolutionary relationships between feline PVs perfectly mirror those of their feline hosts, despite a complex and dynamic phylogeographic history. By applying host species divergence times, we provide the first precise estimates for the rate of evolution for each PV gene, with an overall evolutionary rate of 1.95 x 10(-8) (95% confidence interval 1.32 x 10(-8) to 2.47 x 10(-8)) nucleotide substitutions per site per year for the viral coding genome.
Conclusion: Our work provides evidence for long-term virus-host co-speciation of feline PVs, indicating that viral diversity in slowly evolving viruses can be used to investigate host species evolution. These findings, however, should not be extrapolated to other viral lineages without prior confirmation of virus-host co-divergence.
Figures


References
-
- Drummond AJ, Pybus OG, Rambaut A, Forsberg R, Rodrigo AG. Measurably evolving populations. Trends Ecol Evol. 2003;18:481–488. doi: 10.1016/S0169-5347(03)00216-7. - DOI
-
- Sundberg JP, Montali RJ, Bush M, Phillips LG, O'Brien SJ, Jenson AB, Burk RD, Van Ranst M. Papillomavirus-associated focal oral hyperplasia in wild and captive Asian lions (Panthera leo persica). J Zoo Wildlife Med. 1996;27:61–70.
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

