IMRT in oral cavity cancer
- PMID: 17430599
- PMCID: PMC1855346
- DOI: 10.1186/1748-717X-2-16
IMRT in oral cavity cancer
Abstract
Background: Except for early T1,2 N0 stages, the prognosis for patients with oral cavity cancer (OCC) is reported to be worse than for carcinoma in other sites of the head and neck (HNC). The aim of this work was to assess disease outcome in OCC following IMRT.Between January 2002 and January 2007, 346 HNC patients have been treated with curative intensity modulated radiation therapy (IMRT) at the Department of Radiation Oncology, University Hospital Zurich. Fifty eight of these (16%) were referred for postoperative (28) or definitive (30) radiation therapy of OCC.40 of the 58 OCC patients (69%) presented with locally advanced T3/4 or recurred lesions. Doses between 60 and 70 Gy were applied, combined with simultaneous cisplatin based chemotherapy in 78%. Outcome analyses were performed using Kaplan Meier curves.In addition, comparisons were performed between this IMRT OCC cohort and historic in-house cohorts of 33 conventionally irradiated (3DCRT) and 30 surgery only patients treated over the last 10 years.
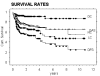
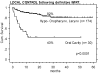
Results: OCC patients treated with postoperative IMRT showed the highest local control (LC) rate of all assessed treatment sequence subgroups (92% LC at 2 years). Historic postoperative 3DCRT patients and patients treated with surgery alone reached LC rates of approximately 70-80%. Definitively irradiated patients revealed poorest LC rates with approximately 30 and 40% following 3DCRT and IMRT, respectively.T1 stage resulted in an expectedly significantly higher LC rate (95%, n = 19, p < 0.05) than T2-4 and recurred stages (LC approximately 50-60%, n = 102).Analyses according to the diagnosis revealed significantly lower LC in OCC following definitive IMRT than that in pharyngeal tumors treated with definitive IMRT in the same time period (43% vs 82% at 2 years, p < 0.0001), while the LC rate of OCC following postoperative IMRT was as high as in pharyngeal tumors treated with postoperative IMRT (>90% at 2 years).
Conclusion: Postoperative IMRT of OCC resulted in the highest local control rate of the assessed treatment subgroups. In conclusion, generous indication for IMRT following surgical treatment is recommended in OCC cases with unfavourable features like tight surgical margin, nodal involvement, primary tumor stage >T1N0, or already recurred disease, respectively.Loco-regional outcome of OCC following definitive IMRT remained unsatisfactory, comparable to that following definitive 3DCRT.
Figures


References
-
- Pimenta Amaral TM, Da Silva Freire AR, Carvalho AL, Pinto CA, Kowalski LP. Predictive factors of occult metastasis and prognosis of clinical stages I and II squamous cell carcinoma of the tongue and floor of the mouth. Oral Oncol. 2004;40:780–786. doi: 10.1016/j.oraloncology.2003.10.009. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

