Identification of the special pair of photosystem II in a chlorophyll d-dominated cyanobacterium
- PMID: 17431035
- PMCID: PMC1851883
- DOI: 10.1073/pnas.0701847104
Identification of the special pair of photosystem II in a chlorophyll d-dominated cyanobacterium
Abstract
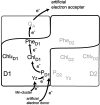
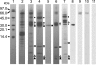
The composition of photosystem II (PSII) in the chlorophyll (Chl) d-dominated cyanobacterium Acaryochloris marina MBIC 11017 was investigated to enhance the general understanding of the energetics of the PSII reaction center. We first purified photochemically active complexes consisting of a 47-kDa Chl protein (CP47), CP43' (PcbC), D1, D2, cytochrome b(559), PsbI, and a small polypeptide. The pigment composition per two pheophytin (Phe) a molecules was 55 +/- 7 Chl d, 3.0 +/- 0.4 Chl a, 17 +/- 3 alpha-carotene, and 1.4 +/- 0.2 plastoquinone-9. The special pair was detected by a reversible absorption change at 713 nm (P713) together with a cation radical band at 842 nm. FTIR difference spectra of the specific bands of a 3-formyl group allowed assignment of the special pair. The combined results indicate that the special pair comprises a Chl d homodimer. The primary electron acceptor was shown by photoaccumulation to be Phe a, and its potential was shifted to a higher value than that in the Chl a/Phe a system. The overall energetics of PSII in the Chl d system are adjusted to changes in the redox potentials, with P713 as the special pair using a lower light energy at 713 nm. Taking into account the reported downward shift in the potential of the special pair of photosystem I (P740) in A. marina, our findings lend support to the idea that changes in photosynthetic pigments combine with a modification of the redox potentials of electron transfer components to give rise to an energetic adjustment of the total reaction system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
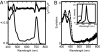

 O stretching and chlorin ring vibrations (B), and 2,770–2,660 cm−1, the region involving the CH stretching vibration of the 3-formyl group (C). Spectra were recorded at 250 K.
O stretching and chlorin ring vibrations (B), and 2,770–2,660 cm−1, the region involving the CH stretching vibration of the 3-formyl group (C). Spectra were recorded at 250 K.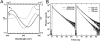
Similar articles
-
Characterization of highly purified photosystem I complexes from the chlorophyll d-dominated cyanobacterium Acaryochloris marina MBIC 11017.J Biol Chem. 2008 Jun 27;283(26):18198-209. doi: 10.1074/jbc.M801805200. Epub 2008 May 5. J Biol Chem. 2008. PMID: 18458090
-
The nature of the photosystem II reaction centre in the chlorophyll d-containing prokaryote, Acaryochloris marina.Photochem Photobiol Sci. 2005 Dec;4(12):1060-4. doi: 10.1039/b507057k. Epub 2005 Nov 7. Photochem Photobiol Sci. 2005. PMID: 16307123
-
Constitution and energetics of photosystem I and photosystem II in the chlorophyll d-dominated cyanobacterium Acaryochloris marina.J Photochem Photobiol B. 2011 Jul-Aug;104(1-2):333-40. doi: 10.1016/j.jphotobiol.2011.02.017. Epub 2011 Feb 23. J Photochem Photobiol B. 2011. PMID: 21530298 Review.
-
Modified molecular interactions of the pheophytin and plastoquinone electron acceptors in photosystem II of chlorophyll D-containing Acaryochloris marina as revealed by FTIR spectroscopy.Photosynth Res. 2015 Aug;125(1-2):105-14. doi: 10.1007/s11120-014-0073-x. Epub 2015 Jan 6. Photosynth Res. 2015. PMID: 25560630
-
Excitation energy transfer in intact cells and in the phycobiliprotein antennae of the chlorophyll d containing cyanobacterium Acaryochloris marina.J Plant Physiol. 2011 Aug 15;168(12):1473-87. doi: 10.1016/j.jplph.2011.02.002. Epub 2011 Mar 10. J Plant Physiol. 2011. PMID: 21396735 Review.
Cited by
-
Dimeric Corrole Analogs of Chlorophyll Special Pairs.J Am Chem Soc. 2021 Jun 30;143(25):9450-9460. doi: 10.1021/jacs.1c02362. Epub 2021 May 20. J Am Chem Soc. 2021. PMID: 34014656 Free PMC article.
-
Redox potentials of primary electron acceptor quinone molecule (QA)- and conserved energetics of photosystem II in cyanobacteria with chlorophyll a and chlorophyll d.Proc Natl Acad Sci U S A. 2011 May 10;108(19):8054-8. doi: 10.1073/pnas.1100173108. Epub 2011 Apr 26. Proc Natl Acad Sci U S A. 2011. PMID: 21521792 Free PMC article.
-
Rapid TaqMan-based quantification of chlorophyll d-containing cyanobacteria in the genus Acaryochloris.Appl Environ Microbiol. 2014 May;80(10):3244-9. doi: 10.1128/AEM.00334-14. Epub 2014 Mar 14. Appl Environ Microbiol. 2014. PMID: 24632258 Free PMC article.
-
Phycobilisomes and Phycobiliproteins in the Pigment Apparatus of Oxygenic Photosynthetics: From Cyanobacteria to Tertiary Endosymbiosis.Int J Mol Sci. 2023 Jan 24;24(3):2290. doi: 10.3390/ijms24032290. Int J Mol Sci. 2023. PMID: 36768613 Free PMC article. Review.
-
A novel species of the marine cyanobacterium Acaryochloris with a unique pigment content and lifestyle.Sci Rep. 2018 Jun 14;8(1):9142. doi: 10.1038/s41598-018-27542-7. Sci Rep. 2018. PMID: 29904088 Free PMC article.
References
-
- Miyashita H, Ikemoto H, Kurano N, Adachi K, Chihara M, Miyachi S. Nature. 1996;383:402.
-
- Murakami A, Miyashita H, Iseki M, Adachi K, Mimuro M. Science. 2004;303:1633. - PubMed
-
- Kühl M, Chen M, Ralph PJ, Schreiber U, Larkum AWD. Nature. 2005;433:820. - PubMed
-
- Miyashita H, Adachi K, Kurano N, Ikemoto H, Chihara M, Miyachi S. Plant Cell Physiol. 1997;38:274–281.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

