Class B alkaline stabilization to achieve pathogen inactivation
- PMID: 17431316
- PMCID: PMC3719960
- DOI: 10.3390/ijerph2007010009
Class B alkaline stabilization to achieve pathogen inactivation
Abstract
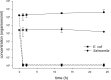
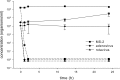
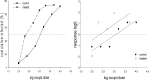
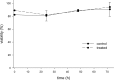
Liming is a cost-effective treatment currently employed in many Class B biosolids production plants in the United States. A bench scale model of lime stabilization was designed to evaluate the persistence of viral, bacterial and parasitic pathogens. The survival of fecal coliforms, Salmonella, adenovirus type 5, rotavirus Wa, bacteriophage MS-2, Cryptosporidium parvum oocysts, Giardia lamblia cysts, and Ascaris lumbricoides ova was evaluated under lime stabilization conditions in a water matrix. Fecal coliforms and Salmonella were undetectable following 2 hours of lime stabilization, demonstrating a 7-log reduction. Adenovirus, MS-2 and rotavirus were below detectable levels following 2 h of liming, demonstrating a 4-log reduction. G. lamblia cysts were also inactivated. A. lumbricoides ova remained viable following 72 hours of liming as did C. parvum oocysts. While this study confirmed that Ascaris ova are resistant to liming, their scarcity in sludge and low recovery efficiencies limit their use as indicator. The persistence of C. parvum oocysts after exposure to lime, suggests that this parasite would be a better choice as indicator for evaluating biosolids intended for land application. The studies done with adenovirus Type 5, rotavirus Wa and male specific bacteriophage provided preliminary data demonstrating similar inactivation rates. Monitoring anthropogenic viruses is a time consuming, labor intensive and expensive process. If further studies could demonstrate that phage could be used as an indicator of other enteric viruses, enhanced monitoring could result in greater acceptance of land application of biosolids while demonstrating no increased public health threat.
Figures
Similar articles
-
Inactivation of adenovirus type 5, rotavirus Wa and male specific coliphage (MS2) in biosolids by lime stabilization.Int J Environ Res Public Health. 2007 Mar;4(1):61-7. doi: 10.3390/ijerph2007010010. Int J Environ Res Public Health. 2007. PMID: 17431317 Free PMC article.
-
Liming as an advanced treatment for sludge sanitisation: helminth eggs elimination--Ascaris eggs as model.Water Res. 2004 Aug-Sep;38(14-15):3251-8. doi: 10.1016/j.watres.2004.04.015. Water Res. 2004. PMID: 15276741
-
Evaluation of solar photocatalysis using TiO2 slurry in the inactivation of Cryptosporidium parvum oocysts in water.J Photochem Photobiol B. 2016 Oct;163:92-9. doi: 10.1016/j.jphotobiol.2016.08.016. Epub 2016 Aug 12. J Photochem Photobiol B. 2016. PMID: 27543761
-
Drinking water treatment processes for removal of Cryptosporidium and Giardia.Vet Parasitol. 2004 Dec 9;126(1-2):219-34. doi: 10.1016/j.vetpar.2004.09.002. Vet Parasitol. 2004. PMID: 15567586 Review.
-
Potential molecular tools for assessing the public health risk associated with waterborne Cryptosporidium oocysts.J Med Microbiol. 2012 Aug;61(Pt 8):1039-1051. doi: 10.1099/jmm.0.043158-0. Epub 2012 May 24. J Med Microbiol. 2012. PMID: 22628454 Review.
Cited by
-
Inactivation of adenovirus type 5, rotavirus Wa and male specific coliphage (MS2) in biosolids by lime stabilization.Int J Environ Res Public Health. 2007 Mar;4(1):61-7. doi: 10.3390/ijerph2007010010. Int J Environ Res Public Health. 2007. PMID: 17431317 Free PMC article.
-
Accuracy of the evaluation method for alkaline agents' bactericidal efficacies in solid, and the required time of bacterial inactivation.J Vet Med Sci. 2017 Feb 4;79(2):244-247. doi: 10.1292/jvms.16-0553. Epub 2016 Nov 25. J Vet Med Sci. 2017. PMID: 27890906 Free PMC article.
-
Durability of alkaline agents' bactericidal efficacies in litter under field conditions.J Vet Med Sci. 2017 May 3;79(5):815-817. doi: 10.1292/jvms.17-0029. Epub 2017 Mar 19. J Vet Med Sci. 2017. PMID: 28321028 Free PMC article.
-
Bactericidal efficacies of food additive grade calcium hydroxide toward Legionella pneumophila.J Vet Med Sci. 2019 Sep 18;81(9):1318-1325. doi: 10.1292/jvms.19-0098. Epub 2019 Jul 9. J Vet Med Sci. 2019. PMID: 31292348 Free PMC article.
-
Farm use of calcium hydroxide as an effective barrier against pathogens.Sci Rep. 2021 Apr 12;11(1):7941. doi: 10.1038/s41598-021-86796-w. Sci Rep. 2021. PMID: 33846406 Free PMC article.
References
-
- Farrell JB, Smith JE, Jr, Hathaway SW, Dean RB. Lime stabilization of primary sludges. J Water Pollut Control Fed. 1974;46(1):113–22. - PubMed
-
- Qi YN, Gillow S, Herson DS, Dentel SK. Reactivation and/or growth of fecal coliform bacteria during centrifugal dewatering of anaerobically digested biosolids. Water Sci Technol. 2004;50(9):115–20. - PubMed
-
- Sidhu J, Gibbs RA, Ho GE, Unkovich I. Selection of Salmonella typhimurium as an indicator for pathogen regrowth potential in composted biosolids. Lett Appl Microbiol. 1999;29(5):303–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
