Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm
- PMID: 17431489
- PMCID: PMC1849915
- DOI: 10.1289/ehp.9469
Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm
Abstract
Background: Nanotechnology offers great promise in many industrial applications. However, little is known about the health effects of manufactured nanoparticles, the building blocks of nanomaterials.
Objectives: Titanium dioxide (TiO(2)) nanoparticles with a primary size of 2-5 nm have not been studied previously in inhalation exposure models and represent some of the smallest manufactured nanoparticles. The purpose of this study was to assess the toxicity of these nanoparticles using a murine model of lung inflammation and injury.
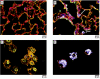
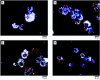
Materials and methods: The properties of TiO(2) nanoparticles as well as the characteristics of aerosols of these particles were evaluated. Mice were exposed to TiO(2) nanoparticles in a whole-body exposure chamber acutely (4 hr) or subacutely (4 hr/day for 10 days). Toxicity in exposed mice was assessed by enumeration of total and differential cells, determination of total protein, lactate dehydrogenase (LDH) activity and inflammatory cytokines in bronchoalveolar lavage (BAL) fluid. Lungs were also evaluated for histopathologic changes
Results: Mice exposed acutely to 0.77 or 7.22 mg/m(3) nanoparticles demonstrated minimal lung toxicity or inflammation. Mice exposed subacutely (8.88 mg/m(3)) and necropsied immediately and at week 1 or 2 postexposure had higher counts of total cells and alveolar macrophages in the BAL fluid compared with sentinels. However, mice recovered by week 3 postexposure. Other indicators were negative.
Conclusions: Mice subacutely exposed to 2-5 nm TiO(2) nanoparticles showed a significant but moderate inflammatory response among animals at week 0, 1, or 2 after exposure that resolved by week 3 postexposure.
Figures



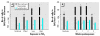
Comment in
-
Aggregation and toxicology of titanium dioxide nanoparticles.Environ Health Perspect. 2008 Apr;116(4):A152; author reply A152-3. doi: 10.1289/ehp.10915R. Environ Health Perspect. 2008. PMID: 18414604 Free PMC article. No abstract available.
References
-
- Anselmann R. Nanoparticles and nanolayers in commercial applications. J Nanoparticle Res. 2001;3:329–336.
-
- Atkins P, de Paula J. 2002. Physical Chemistry. 7th ed. New York:W.H. Freeman and Company, 772–782
-
- Bang JJ, Murr LE. Atmospheric nanoparticles: preliminary studies and potential respiratory health risks for emerging nanotechnologies. J Mater Sci Lett. 2002;21:361–366.
-
- Bermudez E, Mangum J, Wong B, Asgharian B, Hext P, Warheit D, et al. Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol Sci. 2004;77:347–357. - PubMed
-
- Borm PJA. Particle toxicology: from coal mining to nanotechnology. Inhal Toxicol. 2002;14:311–324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

