Differential effects of trisomy on brain shape and volume in related aneuploid mouse models
- PMID: 17431903
- PMCID: PMC3246902
- DOI: 10.1002/ajmg.a.31721
Differential effects of trisomy on brain shape and volume in related aneuploid mouse models
Abstract
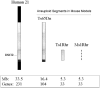
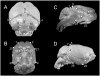
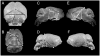
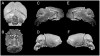
Down syndrome (DS) results from inheritance of three copies of human chromosome 21 (Hsa21). Individuals with DS have a significantly smaller brain size overall and a disproportionately small cerebellum. The small cerebellum is seen in Ts65Dn mice, which have segmental trisomy for orthologs of about half the genes on Hsa21 and provide a genetic model for DS. While small cerebellar size is well-established in mouse and humans, much less is known about the shape of the brain in trisomy. Here we conduct a morphometric analysis of the whole brain and cerebellum in Ts65Dn mice and show that the differences with euploid littermates are largely a function of volume and not of shape. This is not the case in two aneuploid mouse models that have fewer genes orthologous to Hsa21 than Ts65Dn. Ts1Rhr is trisomic for genes corresponding to the so-called Down syndrome critical region (DSCR), which was purported to contain a dosage sensitive gene or genes responsible for many phenotypes of DS. Ms1Rhr is monosomic for the same segment. These models show effects on cerebellum and overall brain that are different from each other and from Ts65Dn. These models can help to identify the contributions of genes from different regions of the chromosome on this and other aspects of brain development in trisomy.
Figures
References
-
- Abrous D, Koehl M, LeMoal M. Adult neurogenesis: From precursors to network and physiology. Physiol Rev. 2005;85:523–569. - PubMed
-
- Arnell H, Gustafsson J, Ivarsson S, Anneren G. Growth and pubertal development in Down syndrome. Acta Paediatr. 1996;85:1102–1106. - PubMed
-
- Aylward E, Habbak R, Warren A, Pulsifer M, Barta P, Jerram M, Pearlson G. Cerebellar volume in adults with Down syndrome. Arch Neurol. 1997;54:209–212. - PubMed
-
- Baxter L, Moran T, Richtsmeier J, Troncoso J, Reeves R. Discovery and genetic localization of Down syndrome cerebellar phenotypes using the Ts65Dn mouse. Hum Mol Genet. 2000;9:195–202. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

