Proteinase-activated receptor 4 stimulation-induced epithelial-mesenchymal transition in alveolar epithelial cells
- PMID: 17433115
- PMCID: PMC1855055
- DOI: 10.1186/1465-9921-8-31
Proteinase-activated receptor 4 stimulation-induced epithelial-mesenchymal transition in alveolar epithelial cells
Abstract
Background: Proteinase-activated receptors (PARs; PAR1-4) that can be activated by serine proteinases such as thrombin and neutrophil catepsin G are known to contribute to the pathogenesis of various pulmonary diseases including fibrosis. Among these PARs, especially PAR4, a newly identified subtype, is highly expressed in the lung. Here, we examined whether PAR4 stimulation plays a role in the formation of fibrotic response in the lung, through alveolar epithelial-mesenchymal transition (EMT) which contributes to the increase in myofibroblast population.
Methods: EMT was assessed by measuring the changes in each specific cell markers, E-cadherin for epithelial cell, alpha-smooth muscle actin (alpha-SMA) for myofibroblast, using primary cultured mouse alveolar epithelial cells and human lung carcinoma-derived alveolar epithelial cell line (A549 cells).
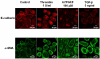
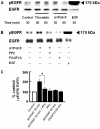
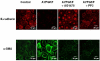
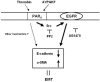
Results: Stimulation of PAR with thrombin (1 U/ml) or a synthetic PAR4 agonist peptide (AYPGKF-NH2, 100 muM) for 72 h induced morphological changes from cobblestone-like structure to elongated shape in primary cultured alveolar epithelial cells and A549 cells. In immunocytochemical analyses of these cells, such PAR4 stimulation decreased E-cadherin-like immunoreactivity and increased alpha-SMA-like immunoreactivity, as observed with a typical EMT-inducer, tumor growth factor-beta (TGF-beta). Western blot analyses of PAR4-stimulated A549 cells also showed similar changes in expression of these EMT-related marker proteins. Such PAR4-mediated changes were attenuated by inhibitors of epidermal growth factor receptor (EGFR) kinase and Src. PAR4-mediated morphological changes in primary cultured alveolar epithelial cells were reduced in the presence of these inhibitors. PAR4 stimulation increased tyrosine phosphorylated EGFR or tyrosine phosphorylated Src level in A549 cells, and the former response being inhibited by Src inhibitor.
Conclusion: PAR4 stimulation of alveolar epithelial cells induced epithelial-mesenchymal transition (EMT) as monitored by cell shapes, and epithelial or myofibroblast marker at least partly through EGFR transactivation via receptor-linked Src activation.
Figures





References
-
- Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Protease-activated receptors. Phamacol Rev. 2001;53:245–282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

