Bacterial response regulators: versatile regulatory strategies from common domains
- PMID: 17433693
- PMCID: PMC3655528
- DOI: 10.1016/j.tibs.2007.03.002
Bacterial response regulators: versatile regulatory strategies from common domains
Abstract
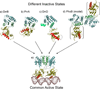
Response regulators (RRs) comprise a major family of signaling proteins in prokaryotes. A modular architecture that consists of a conserved receiver domain and a variable effector domain enables RRs to function as phosphorylation-regulated switches that couple a wide variety of cellular behaviors to environmental cues. Recently, advances have been made in understanding RR functions both at genome-wide and molecular levels. Global techniques have been developed to analyze RR input and output, expanding the scope of characterization of these versatile components. Meanwhile, structural studies have revealed that, despite common structures and mechanisms of function within individual domains, a range of interactions between receiver and effector domains confer great diversity in regulatory strategies, optimizing individual RRs for the specific regulatory needs of different signaling systems.
Figures

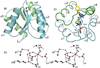

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

