Effect of tricyclodecan-9-yl potassium xanthate (D609) on phospholipid metabolism and cell death during oxygen-glucose deprivation in PC12 cells
- PMID: 17434680
- PMCID: PMC2041837
- DOI: 10.1016/j.neuroscience.2007.02.022
Effect of tricyclodecan-9-yl potassium xanthate (D609) on phospholipid metabolism and cell death during oxygen-glucose deprivation in PC12 cells
Abstract
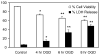
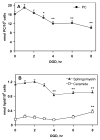
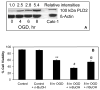
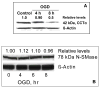
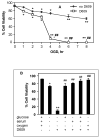
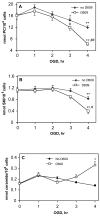
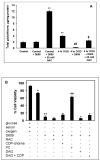
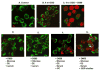
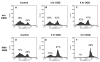
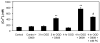
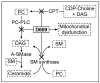
Alterations in lipid metabolism play an integral role in neuronal death in cerebral ischemia. Here we used an in vitro model, oxygen-glucose deprivation (OGD) of rat pheochromocytoma (PC12) cells, and analyzed changes in phosphatidylcholine (PC) and sphingomyelin (SM) metabolism. OGD (4-8 h) of PC12 cells triggered a dramatic reduction in PC and SM levels, and a significant increase in ceramide. OGD also caused increases in phosphatidylcholine-phospholipase C (PC-PLC) and phospholipase D (PLD) activities and PLD2 protein expression, and reduction in cytidine triphosphate:phosphocholine cytidylyltransferase-alpha (CCTalpha, the rate-limiting enzyme in PC synthesis) protein expression and activity. Phospholipase A2 activity and expression were unaltered during OGD. Increased neutral sphingomyelinase activity during OGD could account for SM loss and increased ceramide. Surprisingly, treatment with PC-PLC inhibitor tricyclodecan-9-yl potassium xanthate (D609) aggravated cell death in PC12 cells during OGD. D609 was cytotoxic only during OGD; cell death could be prevented by inclusion of sera, glucose or oxygen. During OGD, D609 caused further loss of PC and SM, depletion of 1,2-diacylglycerol (DAG), increase in ceramide and free fatty acids (FFA), cytochrome c release from mitochondria, increases in intracellular Ca2+ ([Ca2+]i), poly-ADP ribose polymerase (PARP) cleavage and phosphatidylserine externalization, indicative of apoptotic cell death. Exogenous PC during OGD in PC12 cells with D609 attenuated PC, SM loss, restored DAG, attenuated ceramide levels, decreased cytochrome c release, PARP cleavage, annexin V binding, attenuated the increase in [Ca2+]i, FFA release, and significantly increased cell viability. Exogenous PC may have elicited these effects by restoring membrane PC levels. A tentative scheme depicting the mechanism of action of D609 (inhibiting PC-PLC, SM synthase, PC synthesis at the CDP-choline-1,2-diacylglycerol phosphocholine transferase (CPT) step and causing mitochondrial dysfunction) has been proposed based on our observations and literature.
Figures




References
-
- Adibhatla RM, Hatcher JF. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med. 2006;40:376–387. - PubMed
-
- Adibhatla RM, Hatcher JF, Dempsey RJ. Effects of citicoline on phospholipid and glutathione levels in transient cerebral ischemia. Stroke. 2001;32:2376–2381. - PubMed
-
- Adibhatla RM, Hatcher JF, Dempsey RJ. Cytidine-5′-diphosphocholine (CDP-choline) affects CTP:phosphocholine cytidylyltransferase and lyso-phosphatidylcholine after transient brain ischemia. J Neurosci Res. 2004;76:390–396. - PubMed
-
- Adibhatla RM, Hatcher JF, Larsen EC, Chen X, Sun D, Tsao F. CDP-choline significantly restores the phosphatidylcholine levels by differentially affecting phospholipase A2 and CTP-phosphocholine cytidylyltransferase after stroke. J Biol Chem. 2006;281:6718–6725. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

