On kinetics of phage adsorption
- PMID: 17434950
- PMCID: PMC1914437
- DOI: 10.1529/biophysj.106.102962
On kinetics of phage adsorption
Abstract
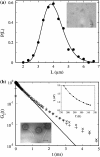
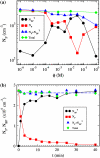
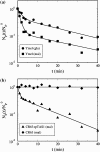
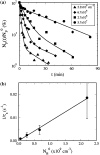
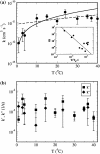
Adsorption of lambda-phage on sensitive bacteria Escherichia coli is a classical problem but not all issues have been resolved. One of the outstanding problems is the rate of adsorption, which in some cases appears to exceed the theoretical limit imposed by the law of random diffusion. We revisit this problem by conducting experiments along with new theoretical analyses. Our measurements show that upon incubating lambda-phage with bacteria Ymel, the population of unbound phage in a salt buffer decreases with time and in general obeys a double-exponential function characterized by a fast (tau(1)) and a slow (tau(2)) decay time. We found that both the fast and the slow processes are specific to interactions between lambda-phage and its receptor LamB. Such specificity motivates a kinetic model that describes the interaction between the phage and the receptor as an on-and-off process followed by an irreversible binding. The latter may be a signature of the initiation of DNA translocation. The kinetic model successfully predicts the double exponential behavior seen in the experiment and allows the corresponding rate constants to be extracted from single measurements. The weak temperature dependence of the reversible and the irreversible binding rate suggests that phage retention by the receptor is entropic in nature and that a molecular key-lock interaction may be an appropriate description of the interaction between the phage tail and the receptor.
Figures


References
-
- Crick, F. 1970. Central dogma of molecular biology. Nature. 227:561–563. - PubMed
-
- Schwartz, M. 1980. Interaction of phages with their receptor proteins. In Virus Receptors (Receptors and Recognition, Series B). L. L. Randall and L. Philipson, editors. Chapman and Hall, London, UK.
-
- Schlesinger, M. 1932. Adsorption of bacteriophages to homologous bacteria. II. Quantitative investigation of adsorption velocity and saturation. Estimation of the particle size of the bacteriophage. Z. Hyg. Infektionskr. 114:149–160.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

