Asymmetric postmating isolation: Darwin's corollary to Haldane's rule
- PMID: 17435235
- PMCID: PMC1894575
- DOI: 10.1534/genetics.106.065979
Asymmetric postmating isolation: Darwin's corollary to Haldane's rule
Abstract
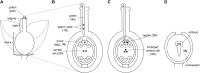
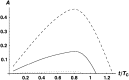
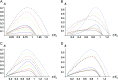
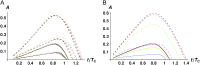
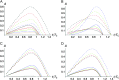
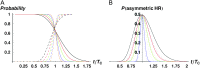
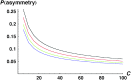
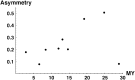
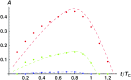
Asymmetric postmating isolation, where reciprocal interspecific crosses produce different levels of fertilization success or hybrid sterility/inviability, is very common. Darwin emphasized its pervasiveness in plants, but it occurs in all taxa assayed. This asymmetry often results from Dobzhansky-Muller incompatibilities (DMIs) involving uniparentally inherited genetic factors (e.g., gametophyte-sporophyte interactions in plants or cytoplasmic-nuclear interactions). Typically, unidirectional (U) DMIs act simultaneously with bidirectional (B) DMIs between autosomal loci that affect reciprocal crosses equally. We model both classes of two-locus DMIs to make quantitative and qualitative predictions concerning patterns of isolation asymmetry in parental species crosses and in the hybrid F(1) generation. First, we find conditions that produce expected differences. Second, we present a stochastic analysis of DMI accumulation to predict probable levels of asymmetry as divergence time increases. We find that systematic interspecific differences in relative rates of evolution for autosomal vs. nonautosomal loci can lead to different expected F(1) fitnesses from reciprocal crosses, but asymmetries are more simply explained by stochastic differences in the accumulation of U DMIs. The magnitude of asymmetry depends primarily on the cumulative effects of U vs. B DMIs (which depend on heterozygous effects of DMIs), the average number of DMIs required to produce complete reproductive isolation (more asymmetry occurs when fewer DMIs are required), and the shape of the function describing how fitness declines as DMIs accumulate. Comparing our predictions to data from diverse taxa indicates that unidirectional DMIs, specifically involving sex chromosomes, cytoplasmic elements, and maternal effects, are likely to play an important role in postmating isolation.
Figures

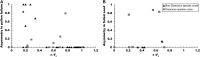
References
-
- Arnold, S. J., P. A. Verrell and S. G. Tilley, 1996. The evolution of asymmetry in sexual isolation: a model and test case. Evolution 50 1024–1033. - PubMed
-
- Bateson, W., 1901. Experiments in plant hybridization. J. R. Hortic. Soc. 26 1–32.
-
- Berlin, S., and H. Ellegren, 2006. Fast accumulation of nonsynonymous mutations on the female-specific W chromosome in birds. J. Mol. Evol. 62 66–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

