Osteopontin has a crucial role in allergic airway disease through regulation of dendritic cell subsets
- PMID: 17435770
- PMCID: PMC3384679
- DOI: 10.1038/nm1580
Osteopontin has a crucial role in allergic airway disease through regulation of dendritic cell subsets
Abstract
Osteopontin (Opn) is important for T helper type 1 (T(H)1) immunity and autoimmunity. However, the role of this cytokine in T(H)2-mediated allergic disease as well as its effects on primary versus secondary antigenic encounters remain unclear. Here we demonstrate that OPN is expressed in the lungs of asthmatic individuals and that Opn-s, the secreted form of Opn, exerts opposing effects on mouse T(H)2 effector responses and subsequent allergic airway disease: pro-inflammatory at primary systemic sensitization, and anti-inflammatory during secondary pulmonary antigenic challenge. These effects of Opn-s are mainly mediated by the regulation of T(H)2-suppressing plasmacytoid dendritic cells (DCs) during primary sensitization and T(H)2-promoting conventional DCs during secondary antigenic challenge. Therapeutic administration of recombinant Opn during pulmonary secondary antigenic challenge decreased established T(H)2 responses and protected mice from allergic disease. These effects on T(H)2 allergic responses suggest that Opn-s is an important therapeutic target and provide new insight into its role in immunity.
Figures
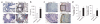




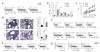
Comment in
-
The bridge between dendritic cells and asthma.Nat Med. 2007 May;13(5):536-8. doi: 10.1038/nm0507-536. Nat Med. 2007. PMID: 17479095 No abstract available.
Similar articles
-
The expression of osteopontin and its association with Clara cell 10 kDa protein in allergic rhinitis.Clin Exp Allergy. 2010 Nov;40(11):1632-41. doi: 10.1111/j.1365-2222.2010.03549.x. Clin Exp Allergy. 2010. PMID: 20553297 Free PMC article.
-
Effects of corticosteroids on osteopontin expression in a murine model of allergic asthma.Int Arch Allergy Immunol. 2009;149 Suppl 1(Suppl 1):7-13. doi: 10.1159/000210647. Epub 2009 Jun 3. Int Arch Allergy Immunol. 2009. PMID: 19494498 Free PMC article.
-
Osteopontin Promotes Protective Antigenic Tolerance against Experimental Allergic Airway Disease.J Immunol. 2018 Feb 15;200(4):1270-1282. doi: 10.4049/jimmunol.1701345. Epub 2018 Jan 12. J Immunol. 2018. PMID: 29330321 Free PMC article.
-
Role of osteopontin in dendritic cell shaping of immune responses.Cytokine Growth Factor Rev. 2019 Dec;50:19-28. doi: 10.1016/j.cytogfr.2019.05.004. Epub 2019 May 10. Cytokine Growth Factor Rev. 2019. PMID: 31126876 Review.
-
Osteopontin and allergic disease: pathophysiology and implications for diagnostics and therapy.Expert Rev Clin Immunol. 2011 Jan;7(1):93-109. doi: 10.1586/eci.10.82. Expert Rev Clin Immunol. 2011. PMID: 21162653 Review.
Cited by
-
Salmeterol attenuates the inflammatory response in asthma and decreases the pro-inflammatory cytokine secretion of dendritic cells.Cell Mol Immunol. 2012 May;9(3):267-75. doi: 10.1038/cmi.2011.56. Epub 2012 Jan 9. Cell Mol Immunol. 2012. PMID: 22231554 Free PMC article.
-
YKL-40 is correlated with FEV1 and the asthma control test (ACT) in asthmatic patients: influence of treatment.BMC Pulm Med. 2015 Jan 12;15:1. doi: 10.1186/1471-2466-15-1. BMC Pulm Med. 2015. PMID: 25578181 Free PMC article.
-
The expression of osteopontin and its association with Clara cell 10 kDa protein in allergic rhinitis.Clin Exp Allergy. 2010 Nov;40(11):1632-41. doi: 10.1111/j.1365-2222.2010.03549.x. Clin Exp Allergy. 2010. PMID: 20553297 Free PMC article.
-
The role of osteopontin in inflammatory processes.J Cell Commun Signal. 2009 Dec;3(3-4):311-22. doi: 10.1007/s12079-009-0068-0. Epub 2009 Oct 2. J Cell Commun Signal. 2009. PMID: 19798593 Free PMC article.
-
Effects of corticosteroids on osteopontin expression in a murine model of allergic asthma.Int Arch Allergy Immunol. 2009;149 Suppl 1(Suppl 1):7-13. doi: 10.1159/000210647. Epub 2009 Jun 3. Int Arch Allergy Immunol. 2009. PMID: 19494498 Free PMC article.
References
-
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986;136:2348–2357. - PubMed
-
- Liu AH. Early intervention for asthma prevention in children. Allergy Asthma Proc. 2002;23:289–293. - PubMed
-
- Weber GF, Cantor H. The immunology of Eta-1/osteopontin. Cytokine Growth Factor Rev. 1996;7:241–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

