Prevalence of factor H-binding protein variants and NadA among meningococcal group B isolates from the United States: implications for the development of a multicomponent group B vaccine
- PMID: 17436227
- PMCID: PMC2245893
- DOI: 10.1086/514821
Prevalence of factor H-binding protein variants and NadA among meningococcal group B isolates from the United States: implications for the development of a multicomponent group B vaccine
Abstract
Background: Two promising recombinant meningococcal protein vaccines are in development. One contains factor H-binding protein (fHBP) variants (v.) 1 and 2, whereas the other contains v.1 and 4 other antigens discovered by genome mining (5 component [5C]). Antibodies against fHBP are bactericidal against strains within a variant group. There are limited data on the prevalence of strains expressing different fHBP variants in the United States.
Methods: A total of 143 group B isolates from patients hospitalized in the United States were tested for fHBP variant by quantitative polymerase chain reaction, for reactivity with 6 anti-fHBP monoclonal antibodies (MAb) by dot immunoblotting, and for susceptibility to bactericidal activity of mouse antisera.
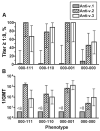
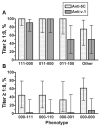
Results: fHBP v.1 isolates predominated in California (83%), whereas isolates expressing v.1 (53%) or v.2 (42%) were common in 9 other states. Isolates representative of 5 anti-fHBP MAb-binding phenotypes (70% of isolates) were highly susceptible to anti-fHBP v.1 or v.2 bactericidal activity, whereas 3 phenotypes were approximately 50% susceptible. Collectively, antibodies against the fHBP v.1 and v.2 vaccine and the 5C vaccine killed 76% and 83% of isolates, respectively.
Conclusions: Susceptibility to bactericidal activity can be predicted, in part, on the basis of fHBP phenotypes. Both vaccines have the potential to prevent most group B disease in the United States.
Conflict of interest statement
Potential conflicts of interest: D.M.G.’s laboratory at Children’s Hospital Oakland Research Institute has research grants from Novartis Vaccines and Diagnostics and from Sanofi Pasteur. D.M.G. also holds a paid consultancy from Novartis. L.H.H. receives research grant support from Sanofi Pasteur and serves as a paid consultant to Sanofi Pasteur, GlaxoSmithKline, and Novartis; he also receives speaking honoraria from Sanofi Pasteur. All other authors declare no conflicts of interest.
Figures



References
-
- Campbell JD, Edelman R, King JC, Jr, Papa T, Ryall R, Rennels MB. Safety, reactogenicity, and immunogenicity of a tetravalent meningococcal polysaccharide–diphtheria toxoid conjugate vaccine given to healthy adults. J Infect Dis. 2002;186:1848–51. - PubMed
-
- Keyserling H, Papa T, Koranyi K, et al. Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents. Arch Pediatr Adolesc Med. 2005;159:907–13. - PubMed
-
- Pichichero M, Casey J, Blatter M, et al. Comparative trial of the safety and immunogenicity of quadrivalent (A, C, Y, W-135) meningococcal polysaccharide-diphtheria conjugate vaccine versus quadrivalent polysaccharide vaccine in two- to ten-year-old children. Pediatr Infect Dis J. 2005;24:57–62. - PubMed
-
- Bilukha OO, Rosenstein N. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention. MMWR Recomm Rep. 2005;54:1–21. - PubMed
-
- Cartwright K, Noah N, Peltola H. Meningococcal disease in Europe: epidemiology, mortality, and prevention with conjugate vaccines. Vaccine; Report of a European advisory board meeting Vienna; Austria. 6–8 October, 2000; 2001. pp. 4347–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

