Mechanistic insights and identification of two novel factors in the C. elegans NMD pathway
- PMID: 17437990
- PMCID: PMC1855233
- DOI: 10.1101/gad.417707
Mechanistic insights and identification of two novel factors in the C. elegans NMD pathway
Abstract
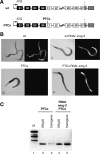
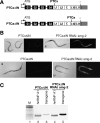
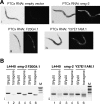
The nonsense-mediated mRNA decay (NMD) pathway selectively degrades mRNAs harboring premature termination codons (PTCs). Seven genes (smg-1-7, for suppressor with morphological effect on genitalia) that are essential for NMD were originally identified in the nematode Caenorhabditis elegans, and orthologs of these genes have been found in several species. Whereas in humans NMD is linked to splicing, PTC definition occurs independently of exon boundaries in Drosophila. Here, we have conducted an analysis of the cis-acting sequences and trans-acting factors that are required for NMD in C. elegans. We show that a PTC codon is defined independently of introns in C. elegans and, consequently, components of the exon junction complex (EJC) are dispensable for NMD. We also show a distance-dependent effect, whereby PTCs that are closer to the 3' end of the mRNA are less sensitive to NMD. We also provide evidence for the existence of previously unidentified components of the NMD pathway that, unlike known smg genes, are essential for viability in C. elegans. A genome-wide RNA interference (RNAi) screen resulted in the identification of two such novel NMD genes, which are essential for proper embryonic development, and as such represent a new class of essential NMD genes in C. elegans that we have termed smgl (for smg lethal). We show that the encoded proteins are conserved throughout evolution and are required for NMD in C. elegans and also in human cells.
Figures




References
-
- Amrani N., Ganesan R., Kervestin S., Mangus D.A., Ghosh S., Jacobson A., Ganesan R., Kervestin S., Mangus D.A., Ghosh S., Jacobson A., Kervestin S., Mangus D.A., Ghosh S., Jacobson A., Mangus D.A., Ghosh S., Jacobson A., Ghosh S., Jacobson A., Jacobson A. A faux 3′-UTR promotes aberrant termination and triggers nonsense-mediated mRNA decay. Nature. 2004;432:112–118. - PubMed
-
- Amrani N., Dong S., He F., Ganesan R., Ghosh S., Kervestin S., Li C., Mangus D.A., Spatrick P., Jacobson A., Dong S., He F., Ganesan R., Ghosh S., Kervestin S., Li C., Mangus D.A., Spatrick P., Jacobson A., He F., Ganesan R., Ghosh S., Kervestin S., Li C., Mangus D.A., Spatrick P., Jacobson A., Ganesan R., Ghosh S., Kervestin S., Li C., Mangus D.A., Spatrick P., Jacobson A., Ghosh S., Kervestin S., Li C., Mangus D.A., Spatrick P., Jacobson A., Kervestin S., Li C., Mangus D.A., Spatrick P., Jacobson A., Li C., Mangus D.A., Spatrick P., Jacobson A., Mangus D.A., Spatrick P., Jacobson A., Spatrick P., Jacobson A., Jacobson A. Aberrant termination triggers nonsense-mediated mRNA decay. Biochem. Soc. Trans. 2006;34:39–42. - PubMed
-
- Azzalin C.M., Lingner J., Lingner J. The human RNA surveillance factor UPF1 is required for S phase progression and genome stability. Curr. Biol. 2006a;16:433–439. - PubMed
-
- Azzalin C.M., Lingner J., Lingner J. The double life of UPF1 in RNA and DNA stability pathways. Cell Cycle. 2006b;5:1496–1498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
