A functional NADPH oxidase prevents caspase involvement in the clearance of phagocytic neutrophils
- PMID: 17438039
- PMCID: PMC1932946
- DOI: 10.1128/IAI.01984-06
A functional NADPH oxidase prevents caspase involvement in the clearance of phagocytic neutrophils
Abstract
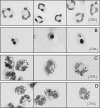
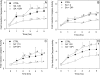
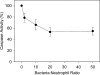
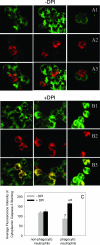
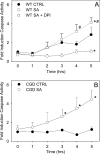
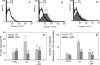
Neutrophils play a prominent role in host defense. Phagocytosis of bacteria leads to the formation of an active NADPH oxidase complex that generates reactive oxygen species for bactericidal purposes. A critical step in the resolution of inflammation is the uptake of neutrophils by macrophages; however, there are conflicting reports on the mechanisms leading to the apoptosis of phagocytic neutrophils. The aim of this study was to clarify the role of effector caspases in these processes. Caspase activity was measured by DEVDase activity assays or immunofluorescence detection of active caspase-3. With normal human and wild-type murine neutrophils there was no caspase activation following phagocytosis of Staphylococcus aureus. However, caspase activity was observed in phagocytic neutrophils with a defective NADPH oxidase, including neutrophils isolated from X-linked gp91(phox) knockout chronic granulomatous disease mice. These results indicate that a functional NADPH oxidase and the generation of oxidants in the neutrophil phagosome prevent the activation of the cytoplasmic caspase cascade.
Figures
References
-
- Borjesson, D. L., S. D. Kobayashi, A. R. Whitney, J. M. Voyich, C. M. Argue, and F. R. Deleo. 2005. Insights into pathogen immune evasion mechanisms: Anaplasma phagocytophilum fails to induce an apoptosis differentiation program in human neutrophils. J. Immunol. 174:6364-6372. - PubMed
-
- Brach, M. A., S. deVos, H. J. Gruss, and F. Herrmann. 1992. Prolongation of survival of human polymorphonuclear neutrophils by granulocyte-macrophage colony-stimulating factor is caused by inhibition of programmed cell death. Blood 80:2920-2924. - PubMed
-
- Cheah, F. C., M. B. Hampton, B. A. Darlow, C. C. Winterbourn, and M. C. Vissers. 2005. Detection of apoptosis by caspase-3 activation in tracheal aspirate neutrophils from premature infants: relationship with NF-kappaB activation. J. Leukoc. Biol. 77:432-437. - PubMed
-
- Colotta, F., F. Re, N. Polentarutti, S. Sozzani, and A. Mantovani. 1992. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 80:2012-2020. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

