The UNC-45 chaperone mediates sarcomere assembly through myosin degradation in Caenorhabditis elegans
- PMID: 17438072
- PMCID: PMC2064129
- DOI: 10.1083/jcb.200607084
The UNC-45 chaperone mediates sarcomere assembly through myosin degradation in Caenorhabditis elegans
Abstract
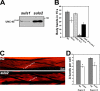
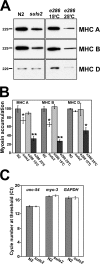
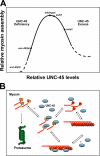
Myosin motors are central to diverse cellular processes in eukaryotes. Homologues of the myosin chaperone UNC-45 have been implicated in the assembly and function of myosin-containing structures in organisms from fungi to humans. In muscle, the assembly of sarcomeric myosin is regulated to produce stable, uniform thick filaments. Loss-of-function mutations in Caenorhabditis elegans UNC-45 lead to decreased muscle myosin accumulation and defective thick filament assembly, resulting in paralyzed animals. We report that transgenic worms overexpressing UNC-45 also display defects in myosin assembly, with decreased myosin content and a mild paralysis phenotype. We find that the reduced myosin accumulation is the result of degradation through the ubiquitin/proteasome system. Partial proteasome inhibition is able to restore myosin protein and worm motility to nearly wild-type levels. These findings suggest a mechanism in which UNC-45-related proteins may contribute to the degradation of myosin in conditions such as heart failure and muscle wasting.
Figures

References
-
- Barral, J.M., A.H. Hutagalung, A. Brinker, F.U. Hartl, and H.F. Epstein. 2002. Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science. 295:669–671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

