Partitioning of amino acid side chains into lipid bilayers: results from computer simulations and comparison to experiment
- PMID: 17438118
- PMCID: PMC2154372
- DOI: 10.1085/jgp.200709745
Partitioning of amino acid side chains into lipid bilayers: results from computer simulations and comparison to experiment
Figures
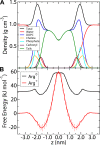
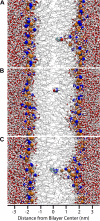
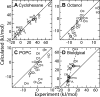
Comment in
-
Lonely arginine seeks friendly environment.J Gen Physiol. 2007 Aug;130(2):233-6. doi: 10.1085/jgp.200709819. Epub 2007 Jul 16. J Gen Physiol. 2007. PMID: 17635960 Free PMC article. No abstract available.
-
Concerning tryptophan and protein-bilayer interactions.J Gen Physiol. 2007 Aug;130(2):223-4. doi: 10.1085/jgp.200709829. Epub 2007 Jul 16. J Gen Physiol. 2007. PMID: 17635961 Free PMC article. No abstract available.
-
Bilayers as protein solvents: role of bilayer structure and elastic properties.J Gen Physiol. 2007 Aug;130(2):225-7. doi: 10.1085/jgp.200709841. Epub 2007 Jul 16. J Gen Physiol. 2007. PMID: 17635962 Free PMC article. No abstract available.
-
Using model membrane-inserted hydrophobic helices to study the equilibrium between transmembrane and nontransmembrane states.J Gen Physiol. 2007 Aug;130(2):229-32. doi: 10.1085/jgp.200709842. Epub 2007 Jul 16. J Gen Physiol. 2007. PMID: 17635963 Free PMC article. No abstract available.
-
Modeling charged protein side chains in lipid membranes.J Gen Physiol. 2007 Aug;130(2):237-40. doi: 10.1085/jgp.200709850. Epub 2007 Jul 16. J Gen Physiol. 2007. PMID: 17635964 Free PMC article. No abstract available.

