Analysis of the assembly profiles for mitochondrial- and nuclear-DNA-encoded subunits into complex I
- PMID: 17438127
- PMCID: PMC1900046
- DOI: 10.1128/MCB.00074-07
Analysis of the assembly profiles for mitochondrial- and nuclear-DNA-encoded subunits into complex I
Abstract
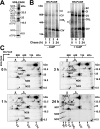
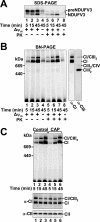
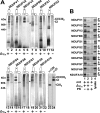
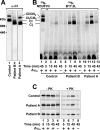
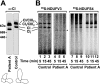
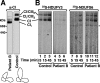
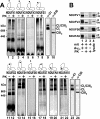
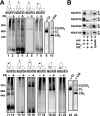
Complex I of the respiratory chain is composed of at least 45 subunits that assemble together at the mitochondrial inner membrane. Defects in human complex I result in energy generation disorders and are also implicated in Parkinson's disease and altered apoptotic signaling. The assembly of this complex is poorly understood and is complicated by its large size and its regulation by two genomes, with seven subunits encoded by mitochondrial DNA (mtDNA) and the remainder encoded by nuclear genes. Here we analyzed the assembly of a number of mtDNA- and nuclear-gene-encoded subunits into complex I. We found that mtDNA-encoded subunits first assemble into intermediate complexes and require significant chase times for their integration into the holoenzyme. In contrast, a set of newly imported nuclear-gene-encoded subunits integrate with preexisting complex I subunits to form intermediates and/or the fully assembly holoenzyme. One of the intermediate complexes represents a subassembly associated with the chaperone B17.2L. By using isolated patient mitochondria, we show that this subassembly is a productive intermediate in complex I assembly since import of the missing subunit restores complex I assembly. Our studies point to a mechanism of complex I biogenesis involving two complementary processes, (i) synthesis of mtDNA-encoded subunits to seed de novo assembly and (ii) exchange of preexisting subunits with newly imported ones to maintain complex I homeostasis. Subunit exchange may also act as an efficient mechanism to prevent the accumulation of oxidatively damaged subunits that would otherwise be detrimental to mitochondrial oxidative phosphorylation and have the potential to cause disease.
Figures
References
-
- Alconada, A., F. Gartner, A. Honlinger, M. Kubrich, and N. Pfanner. 1995. Mitochondrial receptor complex from Neurospora crassa and Saccharomyces cerevisiae. Methods Enzymol. 260:263-286. - PubMed
-
- Antonicka, H., I. Ogilvie, T. Taivassalo, R. P. Anitori, R. G. Haller, J. Vissing, N. G. Kennaway, and E. A. Shoubridge. 2003. Identification and characterization of a common set of complex I assembly intermediates in mitochondria from patients with complex I deficiency. J. Biol. Chem. 278:43081-43088. - PubMed
-
- Carroll, J., I. M. Fearnley, R. J. Shannon, J. Hirst, and J. E. Walker. 2003. Analysis of the subunit composition of complex I from bovine heart mitochondria. Mol. Cell. Proteomics 2:117-126. - PubMed
-
- Carroll, J., I. M. Fearnley, J. M. Skehel, R. J. Shannon, J. Hirst, and J. E. Walker. 2006. Bovine complex I is a complex of forty-five different subunits. J. Biol. Chem. 281:32724-32727. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
