Osteoblast autonomous Pi regulation via Pit1 plays a role in bone mineralization
- PMID: 17438129
- PMCID: PMC1900051
- DOI: 10.1128/MCB.00104-07
Osteoblast autonomous Pi regulation via Pit1 plays a role in bone mineralization
Abstract
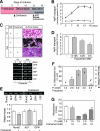
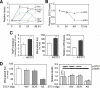
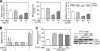
The complex pathogenesis of mineralization defects seen in inherited and/or acquired hypophosphatemic disorders suggests that local inorganic phosphate (P(i)) regulation by osteoblasts may be a rate-limiting step in physiological bone mineralization. To test whether an osteoblast autonomous phosphate regulatory system regulates mineralization, we manipulated well-established in vivo and in vitro models to study mineralization stages separately from cellular proliferation/differentiation stages of osteogenesis. Foscarnet, an inhibitor of NaP(i) transport, blocked mineralization of osteoid formation in osteoblast cultures and local mineralization after injection over the calvariae of newborn rats. Mineralization was also down- and upregulated, respectively, with under- and overexpression of the type III NaP(i) transporter Pit1 in osteoblast cultures. Among molecules expressed in osteoblasts and known to be related to P(i) handling, stanniocalcin 1 was identified as an early response gene after foscarnet treatment; it was also regulated by extracellular P(i), and itself increased Pit1 accumulation in both osteoblast cultures and in vivo. These results provide new insights into the functional role of osteoblast autonomous P(i) handling in normal bone mineralization and the abnormalities seen in skeletal tissue in hypophosphatemic disorders.
Figures

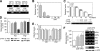

References
-
- ADHR Consortium. 2000. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 26:345-348. - PubMed
-
- Aubin, J. E., J. B. Lain, and G. S. Stein. 2006. Bone formation: maturation and functional activities of osteoblast lineage cells, p. 20-29. In M. J. Favus (ed.), Primer on the metabolic bone diseases and disorders of mineral metabolism, 6th ed. American Society for Bone and Mineral Research, Washington, DC.
-
- Beck, G. R., Jr., and N. Knecht. 2003. Osteopontin regulation by inorganic phosphate is ERK1/2-, protein kinase C-, and proteasome-dependent. J. Biol. Chem. 278:41921-41929. - PubMed
-
- Beck, G. R., Jr. 2003. Inorganic phosphate as a signaling molecule in osteoblast differentiation. J. Cell. Biochem. 90:234-243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
