cPGES/p23 is required for glucocorticoid receptor function and embryonic growth but not prostaglandin E2 synthesis
- PMID: 17438133
- PMCID: PMC1900037
- DOI: 10.1128/MCB.02314-06
cPGES/p23 is required for glucocorticoid receptor function and embryonic growth but not prostaglandin E2 synthesis
Abstract
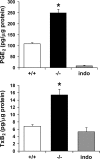
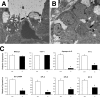
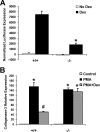
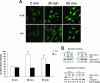
A number of studies have identified cytosolic prostaglandin E(2) synthase (cPGES)/p23 as a cytoplasmic protein capable of metabolism of prostaglandin E(2) (PGE(2)) from the cyclooxygenase metabolite prostaglandin endoperoxide (PGH(2)). However, this protein has also been implicated in a number of other pathways, including stabilization of the glucocorticoid receptor (GR) complex. To define the importance of the functions assigned to this protein, mice lacking detectible cPGES/p23 expression were generated. cPGES/p23(-/-) pups die during the perinatal period and display retarded lung development reminiscent of the phenotype of GR-deficient neonates. Furthermore, GR-sensitive gluconeogenic enzymes are not induced in the prenatal period. However, unlike GR-deficient embryos, cPGES/p23(-/-) embryos are small and a proliferation defect is observed in cPGES/p23(-/-) fibroblasts. Analysis of arachidonic acid metabolites in embryonic tissues and primary fibroblasts failed to support a function for this protein in PGE(2) biosynthesis. Thus, while the growth retardation of the cPGES/p23(-/-) pups and decreased proliferation of primary fibroblasts identify functions for this protein in addition to GR stabilization, it is unlikely that these functions include metabolism of PGH(2) to PGE(2).
Figures







References
-
- Beaudry, J.-B., C. E. Pierreux, G. P. Hayhurst, N. Plumb-Rudewiez, M. C. Weiss, G. G. Rousseau, and F. P. Lemaigre. 2006. Threshold levels of hepatocyte nuclear factor 6 (HNF-6) acting in synergy with HNF-4 and PGC-1α are required for time-specific gene expression during liver development. Mol. Cell. Biol. 26:6037-6046. - PMC - PubMed
-
- Betsholtz, C., L. Karlsson, and P. Lindahl. 2001. Developmental roles of platelet-derived growth factors. BioEssays 23:494-507. - PubMed
-
- Bonventre, J. V., Z. Huang, M. R. Taheri, E. O'Leary, E. Li, M. A. Moskowitz, and A. Sapirstein. 1997. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature 390:622-625. - PubMed
-
- Brewer, J. A., O. Kanagawa, B. P. Sleckman, and L. J. Muglia. 2002. Thymocyte apoptosis induced by T cell activation is mediated by glucocorticoids in vivo. J. Immunol. 169:1837-1843. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
