Foldamers as versatile frameworks for the design and evolution of function
- PMID: 17438550
- PMCID: PMC3810020
- DOI: 10.1038/nchembio876
Foldamers as versatile frameworks for the design and evolution of function
Abstract
Foldamers are sequence-specific oligomers akin to peptides, proteins and oligonucleotides that fold into well-defined three-dimensional structures. They offer the chemical biologist a broad pallet of building blocks for the construction of molecules that test and extend our understanding of protein folding and function. Foldamers also provide templates for presenting complex arrays of functional groups in virtually unlimited geometrical patterns, thereby presenting attractive opportunities for the design of molecules that bind in a sequence- and structure-specific manner to oligosaccharides, nucleic acids, membranes and proteins. We summarize recent advances and highlight the future applications and challenges of this rapidly expanding field.
Conflict of interest statement
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at
Figures
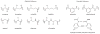
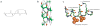





References
-
- DeGrado WF, Summa CM, Pavone V, Nastri F, Lombardi A. De novo design and structural characterization of proteins and metalloproteins. Annu Rev Biochem. 1999;68:779–819. - PubMed
-
- Alvizo O, Allen BD, Mayo SL. Computational protein design promises to revolutionize protein engineering. Biotechniques. 2007;42:31, 33, 35. passim. - PubMed
-
- Gellman SH. Foldamers: a manifesto. Acc Chem Res. 1998;31:173–180.
-
- Rueping M, Mahajan Y, Sauer M, Seebach D. Cellular uptake studies with β-peptides. Chem Bio Chem. 2002;3:257–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

