Electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury
- PMID: 17439349
- PMCID: PMC2435168
- DOI: 10.1089/neu.2006.0011
Electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury
Abstract
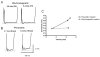
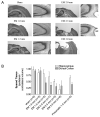
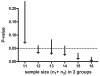
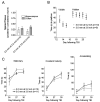
Genetically modified mice represent useful tools for traumatic brain injury (TBI) research and attractive preclinical models for the development of novel therapeutics. Experimental methods that minimize the number of mice needed may increase the pace of discovery. With this in mind, we developed and characterized a prototype electromagnetic (EM) controlled cortical impact device along with refined surgical and behavioral testing techniques. By varying the depth of impact between 1.0 and 3.0 mm, we found that the EM device was capable of producing a broad range of injury severities. Histologically, 2.0-mm impact depth injuries produced by the EM device were similar to 1.0-mm impact depth injuries produced by a commercially available pneumatic device. Behaviorally, 2.0-, 2.5-, and 3.0-mm impacts impaired hidden platform and probe trial water maze performance, whereas 1.5-mm impacts did not. Rotorod and visible platform water maze deficits were also found following 2.5- and 3.0-mm impacts. No impairment of conditioned fear performance was detected. No differences were found between sexes of mice. Inter-operator reliability was very good. Behaviorally, we found that we could statistically distinguish between injury depths differing by 0.5 mm using 12 mice per group and between injury depths differing by 1.0 mm with 7-8 mice per group. Thus, the EM impactor and refined surgical and behavioral testing techniques may offer a reliable and convenient framework for preclinical TBI research involving mice.
Figures



References
-
- ABRAHAMSON EE, IKONOMOVIC MD, CIALLELLA JR, et al. Caspase inhibition therapy abolishes brain trauma-induced increases in Abeta peptide: implications for clinical outcome. Exp. Neurol. 2006;197:437–450. - PubMed
-
- BAYIR H, CLARK RS, KOCHANEK PM. Promising strategies to minimize secondary brain injury after head trauma. Crit. Care Med. 2003;31:S112–S117. - PubMed
-
- BAYIR H, KAGAN VE, BORISENKO GG, et al. Enhanced oxidative stress in iNOS-deficient mice after traumatic brain injury: support for a neuroprotective role of iNOS. J. Cereb. Blood Flow Metab. 2005;25:673–684. - PubMed
-
- BERMPOHL D, YOU Z, KORSMEYER SJ, MOSKOWITZ MA, WHALEN MJ. Traumatic brain injury in mice deficient in Bid: effects on histopathology and functional outcome. J. Cereb. Blood Flow Metab. 2006;26:625–633. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

