Identification of two novel glial-restricted cell populations in the embryonic telencephalon arising from unique origins
- PMID: 17439658
- PMCID: PMC1858687
- DOI: 10.1186/1471-213X-7-33
Identification of two novel glial-restricted cell populations in the embryonic telencephalon arising from unique origins
Abstract
Background: Considerably less attention has been given to understanding the cellular components of gliogenesis in the telencephalon when compared to neuronogenesis, despite the necessity of normal glial cell formation for neurological function. Early proposals of exclusive ventral oligodendrocyte precursor cell (OPC) generation have been challenged recently with studies revealing the potential of the dorsal telencephalon to also generate oligodendrocytes. The identification of OPCs generated from multiple regions of the developing telencephalon, together with the need of the embryonic telencephalon to provide precursor cells for oligodendrocytes as well as astrocytes in ventral and dorsal areas, raises questions concerning the identity of the precursor cell populations capable of generating macroglial subtypes during multiple developmental windows and in differing locations.
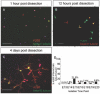
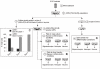
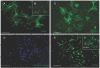
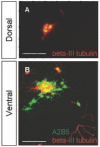
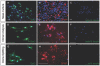
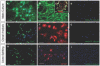
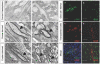
Results: We have identified progenitor populations in the ventral and dorsal telencephalon restricted to the generation of astrocytes and oligodendrocytes. We further demonstrate that the dorsal glial progenitor cells can be generated de novo from the dorsal telencephalon and we demonstrate their capacity for in vivo production of both myelin-forming oligodendrocytes and astrocytes upon transplantation.
Conclusion: Based on our results we offer a unifying model of telencephalic gliogenesis, with the generation of both oligodendrocytes and astrocytes from spatially separate, but functionally similar, glial restricted populations at different developmental times in the dorsal and ventral CNS.
Figures


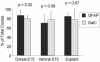


References
-
- Mayer-Proschel M. Cell differentiation in the embryonic mammalian spinal cord. J Neural Transm Suppl. 1999;55:1–8. - PubMed
-
- Gregori N, Proschel C, Noble M, Mayer-Proschel M. The tripotential glial-restricted precursor (GRP) cell and glial development in the spinal cord: generation of bipotential oligodendrocyte-type-2 astrocyte progenitor cells and dorsal-ventral differences in GRP cell function. J Neurosci. 2002;22:248–256. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

