The Rac1 guanine nucleotide exchange factor Tiam1 mediates EphB receptor-dependent dendritic spine development
- PMID: 17440041
- PMCID: PMC1855368
- DOI: 10.1073/pnas.0702044104
The Rac1 guanine nucleotide exchange factor Tiam1 mediates EphB receptor-dependent dendritic spine development
Abstract
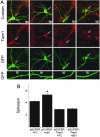
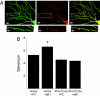
Dendritic spines are small, actin-rich protrusions on the surface of dendrites that receive the majority of excitatory synaptic inputs in the brain. The formation and remodeling of spines, processes that underlie synaptic development and plasticity, are regulated in part by Eph receptor tyrosine kinases. However, the mechanism by which Ephs regulate actin cytoskeletal remodeling necessary for spine development is not fully understood. Here, we report that the Rac1 guanine nucleotide exchange factor Tiam1 interacts with the EphB2 receptor in a kinase-dependent manner. Activation of EphBs by their ephrinB ligands induces the tyrosine phosphorylation and recruitment of Tiam1 to EphB complexes containing NMDA-type glutamate receptors. Either knockdown of Tiam1 protein by RNAi or inhibition of Tiam1 function with a dominant-negative Tiam1 mutant blocks dendritic spine formation induced by ephrinB1 stimulation. Taken together, these findings suggest that EphBs regulate spine development in part by recruiting, phosphorylating, and activating Tiam1. Tiam1 can then promote Rac1-dependent actin cytoskeletal remodeling required for dendritic spine morphogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Harris KM, Kater SB. Annu Rev Neurosci. 1994;17:341–371. - PubMed
-
- Yuste R, Bonhoeffer T. Annu Rev Neurosci. 2001;24:1071–1089. - PubMed
-
- Kaufmann WE, Moser HW. Cereb Cortex. 2000;10:981–991. - PubMed
-
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Trends Neurosci. 2003;26:360–368. - PubMed
-
- Yamaguchi Y, Pasquale EB. Curr Opin Neurobiol. 2004;14:288–296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

