Elucidating the function of secreted maspin: inhibiting cathepsin D-mediated matrix degradation
- PMID: 17440060
- PMCID: PMC3177104
- DOI: 10.1158/0008-5472.CAN-06-4767
Elucidating the function of secreted maspin: inhibiting cathepsin D-mediated matrix degradation
Erratum in
- Cancer Res. 2007 May 15;67(10):5060
Abstract
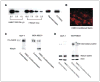
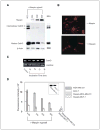
Cellular interaction with the extracellular milieu plays a significant role in normal biological and pathologic processes. Excessive degradation of basement membrane matrix by proteolytic enzymes is a hallmark of tumor invasion and metastasis, and aspartyl proteinase cathepsin D is implicated as a major contributor to this process. Maspin, a non-inhibitory serpin, plays an important role in mammary gland development and remodeling. Expression of Maspin is decreased in primary tumors and lost in metastatic lesions. Maspin is mostly cytoplasmic and is partially secreted; however, the fate and function of secreted Maspin has remained mostly unexplored. We hypothesized that secreted Maspin is incorporated into the matrix deposited by normal mammary epithelial cells and thus could play a critical role in cathepsin D-mediated matrix degradation and remodeling of mammary tissue. In the absence of Maspin, as is the case with most cancer cells, matrix degradation proceeds unrestricted, thus facilitating the progression to metastasis. To test this, we employed an in vitro model where gels containing both types I and IV collagen were preconditioned with normal mammary epithelial cells to allow the incorporation of secreted Maspin. This conditioned matrix was used to examine cathepsin D-mediated collagen degradation by human breast cancer cell lines. Our results indicate that secretion of Maspin and its deposition into the extracellular milieu play an important role in matrix degradation. In this capacity, Maspin could potentially regulate mammary tissue remodeling occurring under normal and pathologic conditions. In addition, these findings could have a potential effect on future therapeutic intervention strategies for breast cancer.
Figures

Similar articles
-
IFN-gamma regulation of vacuolar pH, cathepsin D processing and autophagy in mammary epithelial cells.J Cell Biochem. 2008 Sep 1;105(1):208-18. doi: 10.1002/jcb.21814. J Cell Biochem. 2008. PMID: 18494001 Free PMC article.
-
Multiple functions of maspin in tumor progression and mouse development.Front Biosci. 2004 Sep 1;9:2218-26. doi: 10.2741/1392. Front Biosci. 2004. PMID: 15353283 Review.
-
Maspin regulates hypoxia-mediated stimulation of uPA/uPAR complex in invasive breast cancer cells.Cancer Biol Ther. 2005 Apr;4(4):400-6. doi: 10.4161/cbt.4.4.1617. Epub 2005 Apr 21. Cancer Biol Ther. 2005. PMID: 15846059 Free PMC article.
-
Maspin is physically associated with [beta]1 integrin regulating cell adhesion in mammary epithelial cells.FASEB J. 2006 Jul;20(9):1510-2. doi: 10.1096/fj.05-5500fje. Epub 2006 May 23. FASEB J. 2006. PMID: 16720730
-
The promise and challenge toward the clinical application of maspin in cancer.Front Biosci. 2004 Sep 1;9:2733-45. doi: 10.2741/1432. Front Biosci. 2004. PMID: 15353310 Review.
Cited by
-
Differentiation of the mammary epithelial cell during involution: implications for breast cancer.J Mammary Gland Biol Neoplasia. 2009 Jun;14(2):159-70. doi: 10.1007/s10911-009-9121-0. Epub 2009 May 1. J Mammary Gland Biol Neoplasia. 2009. PMID: 19408104 Review.
-
Maspin, the molecular bridge between the plasminogen activator system and beta1 integrin that facilitates cell adhesion.J Biol Chem. 2011 Jul 15;286(28):24599-607. doi: 10.1074/jbc.M111.235788. Epub 2011 May 23. J Biol Chem. 2011. PMID: 21606500 Free PMC article.
-
Untangling the Extracellular Matrix of Idiopathic Epiretinal Membrane: A Path Winding among Structure, Interactomics and Translational Medicine.Cells. 2022 Aug 15;11(16):2531. doi: 10.3390/cells11162531. Cells. 2022. PMID: 36010606 Free PMC article. Review.
-
Two Faces of Cathepsin D: Physiological Guardian Angel and Pathological Demon.Biol Med (Aligarh). 2014 Jul;6(2):1000206. doi: 10.4172/0974-8369.1000206. Biol Med (Aligarh). 2014. PMID: 25663755 Free PMC article.
-
IFN-gamma regulation of vacuolar pH, cathepsin D processing and autophagy in mammary epithelial cells.J Cell Biochem. 2008 Sep 1;105(1):208-18. doi: 10.1002/jcb.21814. J Cell Biochem. 2008. PMID: 18494001 Free PMC article.
References
-
- Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284:67–8. - PubMed
-
- Ren WP, Sloane BF. Cathepsin D and B in breast cancer. Cancer Treat Res. 1996;83:325–52. - PubMed
-
- Capony F, Rougeot C, Montcourrier P, Cavailles V, Salazar G, Rochefort H. Increased secretion, altered processing, and glycosylation of pro-cathepsin D in human mammary cancer cells. Cancer Res. 1989;49:3904–9. - PubMed
-
- Dittmer F, Pohlmann R, von Figura K. The phosphorylation pattern of oligosaccharides in secreted procathepsin D is glycosylation site-specific and independent of the expression of mannose-6-phosphate receptors. J Biol Chem. 1997;272:852–8. - PubMed
-
- Liaudet-Coopman E, Beaujouin M, Derocq D, et al. Cathepsin D: newly discovered functions of a longstanding aspartic protease in cancer and apoptosis. Cancer Lett. 2006;237:167–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

