Evolution of axis specification mechanisms in jawed vertebrates: insights from a chondrichthyan
- PMID: 17440610
- PMCID: PMC1847705
- DOI: 10.1371/journal.pone.0000374
Evolution of axis specification mechanisms in jawed vertebrates: insights from a chondrichthyan
Abstract
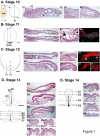
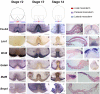
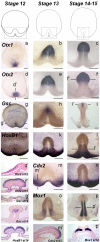
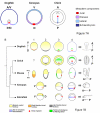
The genetic mechanisms that control the establishment of early polarities and their link with embryonic axis specification and patterning seem to substantially diverge across vertebrates. In amphibians and teleosts, the establishment of an early dorso-ventral polarity determines both the site of axis formation and its rostro-caudal orientation. In contrast, amniotes retain a considerable plasticity for their site of axis formation until blastula stages and rely on signals secreted by extraembryonic tissues, which have no clear equivalents in the former, for the establishment of their rostro-caudal pattern. The rationale for these differences remains unknown. Through detailed expression analyses of key development genes in a chondrichthyan, the dogfish Scyliorhinus canicula, we have reconstructed the ancestral pattern of axis specification in jawed vertebrates. We show that the dogfish displays compelling similarities with amniotes at blastula and early gastrula stages, including the presence of clear homologs of the hypoblast and extraembryonic ectoderm. In the ancestral state, these territories are specified at opposite poles of an early axis of bilateral symmetry, homologous to the dorso-ventral axis of amphibians or teleosts, and aligned with the later forming embryonic axis, from head to tail. Comparisons with amniotes suggest that a dorsal expansion of extraembryonic ectoderm, resulting in an apparently radial symmetry at late blastula stages, has taken place in their lineage. The synthesis of these results with those of functional analyses in model organisms supports an evolutionary link between the dorso-ventral polarity of amphibians and teleosts and the embryonic-extraembryonic organisation of amniotes. It leads to a general model of axis specification in gnathostomes, which provides a comparative framework for a reassessment of conservations both among vertebrates and with more distant metazoans.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

