Mechanisms of TSC-mediated control of synapse assembly and axon guidance
- PMID: 17440611
- PMCID: PMC1847706
- DOI: 10.1371/journal.pone.0000375
Mechanisms of TSC-mediated control of synapse assembly and axon guidance
Abstract
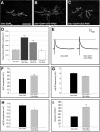
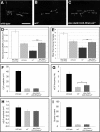
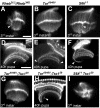
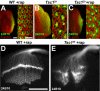
Tuberous sclerosis complex is a dominant genetic disorder produced by mutations in either of two tumor suppressor genes, TSC1 and TSC2; it is characterized by hamartomatous tumors, and is associated with severe neurological and behavioral disturbances. Mutations in TSC1 or TSC2 deregulate a conserved growth control pathway that includes Ras homolog enriched in brain (Rheb) and Target of Rapamycin (TOR). To understand the function of this pathway in neural development, we have examined the contributions of multiple components of this pathway in both neuromuscular junction assembly and photoreceptor axon guidance in Drosophila. Expression of Rheb in the motoneuron, but not the muscle of the larval neuromuscular junction produced synaptic overgrowth and enhanced synaptic function, while reductions in Rheb function compromised synapse development. Synapse growth produced by Rheb is insensitive to rapamycin, an inhibitor of Tor complex 1, and requires wishful thinking, a bone morphogenetic protein receptor critical for functional synapse expansion. In the visual system, loss of Tsc1 in the developing retina disrupted axon guidance independently of cellular growth. Inhibiting Tor complex 1 with rapamycin or eliminating the Tor complex 1 effector, S6 kinase (S6k), did not rescue axon guidance abnormalities of Tsc1 mosaics, while reductions in Tor function suppressed those phenotypes. These findings show that Tsc-mediated control of axon guidance and synapse assembly occurs via growth-independent signaling mechanisms, and suggest that Tor complex 2, a regulator of actin organization, is critical in these aspects of neuronal development.
Conflict of interest statement
Figures



References
-
- Ess KC. The neurobiology of tuberous sclerosis complex. Semin Pediatr Neurol. 2006;13:37–42. - PubMed
-
- Tavazoie SF, Alvarez VA, Ridenour DA, Kwiatkowski DJ, Sabatini BL. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat Neurosci. 2005;8:1727–1734. - PubMed
-
- von der Brelie C, Waltereit R, Zhang L, Beck H, Kirschstein T. Impaired synaptic plasticity in a rat model of tuberous sclerosis. Eur J Neurosci. 2006;23:686–692. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

