Homocysteine affects cardiomyocyte viability: concentration-dependent effects on reversible flip-flop, apoptosis and necrosis
- PMID: 17440815
- PMCID: PMC1914234
- DOI: 10.1007/s10495-007-0077-5
Homocysteine affects cardiomyocyte viability: concentration-dependent effects on reversible flip-flop, apoptosis and necrosis
Abstract
Background: Hyperhomocysteinaemia (HHC) is thought to be a risk factor for cardiovascular disease including heart failure. While numerous studies have analyzed the role of homocysteine (Hcy) in the vasculature, only a few studies investigated the role of Hcy in the heart. Therefore we have analyzed the effects of Hcy on isolated cardiomyocytes.
Methods: H9c2 cells (rat cardiomyoblast cells) and adult rat cardiomyocytes were incubated with Hcy and were analyzed for cell viability. Furthermore, we determined the effects of Hcy on intracellular mediators related to cell viability in cardiomyocytes, namely NOX2, reactive oxygen species (ROS), mitochondrial membrane potential (DeltaPsi (m)) and ATP concentrations.
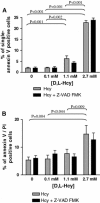
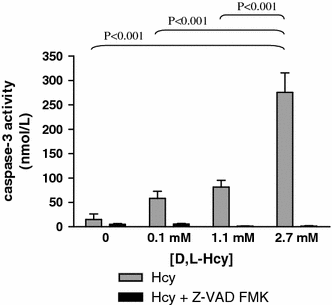
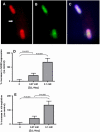
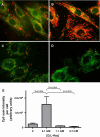
Results: We found that incubation of H9c2 cells with 0.1 mM D,L-Hcy (= 60 microM L-Hcy) resulted in an increase of DeltaPsi (m) as well as ATP concentrations. 1.1 mM D,L-Hcy (= 460 microM L-Hcy) induced reversible flip-flop of the plasma membrane phospholipids, but not apoptosis. Incubation with 2.73 mM D,L-Hcy (= 1.18 mM L-Hcy) induced apoptosis and necrosis. This loss of cell viability was accompanied by a thread-to-grain transition of the mitochondrial reticulum, ATP depletion and nuclear NOX2 expression coinciding with ROS production as evident from the presence of nitrotyrosin residues. Notably, only at this concentration we found a significant increase in S-adenosylhomocysteine which is considered the primary culprit in HHC.
Conclusion: We found concentration-dependent effects of Hcy in cardiomyocytes, varying from induction of reversible flip-flop of the plasma membrane phospholipids, to apoptosis and necrosis.
Figures



References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1056/NEJM199804093381507', 'is_inner': False, 'url': 'https://doi.org/10.1056/nejm199804093381507'}, {'type': 'PubMed', 'value': '9535670', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9535670/'}]}
- Welch GN, Loscalzo J (1998) Homocysteine and atherothrombosis. N Engl J Med 338:1042–1050 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1038/ng0595-111', 'is_inner': False, 'url': 'https://doi.org/10.1038/ng0595-111'}, {'type': 'PubMed', 'value': '7647779', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/7647779/'}]}
- Frosst P, Blom HJ, Milos R et al (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10:111–113 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1161/01.ATV.0000014588.71807.0A', 'is_inner': False, 'url': 'https://doi.org/10.1161/01.atv.0000014588.71807.0a'}, {'type': 'PubMed', 'value': '12006389', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12006389/'}]}
- Symons JD, Mullick AE, Ensunsa JL, Ma AA, Rutledge JC (2002) Hyperhomocysteinemia evoked by folate depletion: effects on coronary and carotid arterial function. Arterioscler Thromb Vasc Biol 22:772–780 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PMC', 'value': 'PMC2013581', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC2013581/'}, {'type': 'PubMed', 'value': '5792556', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/5792556/'}]}
- McCully KS (1969) Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol 56:111–128 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1055/s-2000-8469', 'is_inner': False, 'url': 'https://doi.org/10.1055/s-2000-8469'}, {'type': 'PubMed', 'value': '11011842', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11011842/'}]}
- Coppola A, Davi G, De SV, Mancini FP, Cerbone AM, Di Minno G (2000) Homocysteine, coagulation, platelet function, and thrombosis. Semin Thromb Hemost 26:243–254 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

