A pilot study with a therapeutic vaccine based on hydroxyapatite ceramic particles and self-antigens in cancer patients
- PMID: 17441505
- PMCID: PMC1852891
- DOI: 10.1379/csc-218r.1
A pilot study with a therapeutic vaccine based on hydroxyapatite ceramic particles and self-antigens in cancer patients
Abstract
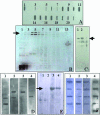
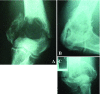
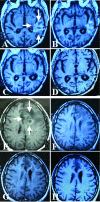
We describe an approach to produce an autologous therapeutic antitumor vaccine using hydroxyapatite (HA) for vaccinating cancer patients. The novel approach involved (1) the purification of part of the self-tumor antigens/ adjuvants using column chromatography with HA, (2) the employ of HA as a medium to attract antigen-presenting cells (APCs) to the vaccination site, and (3) the use of HA as a vector to present in vivo the tumor antigens and adjuvants to the patient's APCs. The vaccine was prepared using and combining HA particles, with at least 3 heat shock proteins (gp96 was one of them possibly with chaperoned proteins/peptides as shown in the slot blots) and with proteins from the cell membrane system (including Hsp70, Hsp27, and membrane proteins). The timing of HA degradation was tested in rats; the HA particles administered under the skin attracted macrophages and were degraded into smaller particles, and they were totally phagocytized within 1 week. In patients (n = 20), the vaccine was then administered weekly and showed very low toxicity, causing minor and tolerable local inflammation (erythema, papule, or local pain); only 1 patient who received a larger dose presented hot flashes, and there were no systemic manifestations of toxicity or autoimmune diseases attributed to the vaccine. Our study suggests that this therapeutic vaccine has shown some efficacy producing a positive response in certain patients. Stable disease was noted in 25% of the patients (renal carcinoma, breast carcinoma, and astrocytoma), and a partial response was noted in 15% of the patients (breast carcinoma and astrocytoma). The most encouraging results were seen in patients with recurrent disease; 4 patients in these conditions (20%) are disease free following the vaccine administration. However, we do not want to overstate the clinical efficacy in this small number of patients. The therapeutic vaccine tested in our study is working by activating the T-cell response as was shown in the comparative histological and immunohistochemical study performed in the pre- and postvaccine biopsy taken from a patient with inflammatory breast carcinoma. However, we cannot ruled out that the vaccine could also be producing an antibody(ies)-mediated response. In conclusion, this therapeutic vaccine based on HA ceramic particles and self-antigens can be safely administered and is showing some encouraging clinical results in cancer patients.
Figures



Similar articles
-
Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: clinical and immunologic findings.J Clin Oncol. 2002 Oct 15;20(20):4169-80. doi: 10.1200/JCO.2002.09.134. J Clin Oncol. 2002. PMID: 12377960 Clinical Trial.
-
Cancer vaccine--Antigenics.BioDrugs. 2002;16(1):72-4. doi: 10.2165/00063030-200216010-00009. BioDrugs. 2002. PMID: 11909004 Review.
-
Phase I study utilizing a novel antigen-presenting cell-targeted vaccine with Toll-like receptor stimulation to induce immunity to self-antigens in cancer patients.Clin Cancer Res. 2011 Jul 15;17(14):4844-53. doi: 10.1158/1078-0432.CCR-11-0891. Epub 2011 Jun 1. Clin Cancer Res. 2011. PMID: 21632857 Free PMC article. Clinical Trial.
-
Immunization of cancer patients with autologous cancer-derived heat shock protein gp96 preparations: a pilot study.Int J Cancer. 2000 Oct 15;88(2):232-8. doi: 10.1002/1097-0215(20001015)88:2<232::aid-ijc14>3.0.co;2-8. Int J Cancer. 2000. PMID: 11004674 Clinical Trial.
-
Heat shock proteins (HSPs) based anti-cancer vaccines.Curr Mol Med. 2012 Nov 1;12(9):1183-97. doi: 10.2174/156652412803306684. Curr Mol Med. 2012. PMID: 22804241 Review.
Cited by
-
Activation of NLRP3 Inflammasome Complexes by Beta-Tricalcium Phosphate Particles and Stimulation of Immune Cell Migration in vivo.J Innate Immun. 2022;14(3):207-217. doi: 10.1159/000518953. Epub 2021 Oct 7. J Innate Immun. 2022. PMID: 34619679 Free PMC article.
-
The Importance of the Tumor Microenvironment and Hypoxia in Delivering a Precision Medicine Approach to Veterinary Oncology.Front Vet Sci. 2020 Nov 12;7:598338. doi: 10.3389/fvets.2020.598338. eCollection 2020. Front Vet Sci. 2020. PMID: 33282935 Free PMC article. Review.
-
APAVAC Immunotherapy for the Adjuvant Treatment of a Canine Mucosal Melanoma.Vet Sci. 2024 Dec 6;11(12):628. doi: 10.3390/vetsci11120628. Vet Sci. 2024. PMID: 39728968 Free PMC article.
-
Azoximer bromide and hydroxyapatite: promising immune adjuvants in cancer.Cancer Biol Med. 2024 Feb 5;20(12):1021-34. doi: 10.20892/j.issn.2095-3941.2023.0222. Cancer Biol Med. 2024. PMID: 38318840 Free PMC article. Review.
-
A phase I study on combined therapy with proton-beam radiotherapy and in situ tumor vaccination for locally advanced recurrent hepatocellular carcinoma.Radiat Oncol. 2013 Oct 16;8:239. doi: 10.1186/1748-717X-8-239. Radiat Oncol. 2013. PMID: 24131485 Free PMC article. Clinical Trial.
References
-
- Belli F, Testori A, and Rivoltini L. et al. 2002 Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: clinical and immunologic findings. J Clin Oncol. 20:4169–4180. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
