Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal
- PMID: 17442830
- PMCID: PMC2680207
- DOI: 10.1523/JNEUROSCI.0134-07.2007
Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal
Abstract
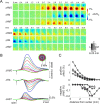
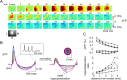
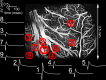
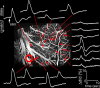
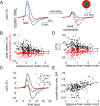
Synaptic transmission initiates a cascade of signal transduction events that couple neuronal activity to local changes in blood flow and oxygenation. Although a number of vasoactive molecules and specific cell types have been implicated, the transformation of stimulus-induced activation of neuronal circuits to hemodynamic changes is still unclear. We use somatosensory stimulation and a suite of in vivo imaging tools to study neurovascular coupling in rat primary somatosensory cortex. Our stimulus evoked a central region of net neuronal depolarization surrounded by net hyperpolarization. Hemodynamic measurements revealed that predominant depolarization corresponded to an increase in oxygenation, whereas predominant hyperpolarization corresponded to a decrease in oxygenation. On the microscopic level of single surface arterioles, the response was composed of a combination of dilatory and constrictive phases. Critically, the relative strength of vasoconstriction covaried with the relative strength of oxygenation decrease and neuronal hyperpolarization. These results suggest that a neuronal inhibition and concurrent arteriolar vasoconstriction correspond to a decrease in blood oxygenation, which would be consistent with a negative blood oxygenation level-dependent functional magnetic resonance imaging signal.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
