AKAP12 regulates human blood-retinal barrier formation by downregulation of hypoxia-inducible factor-1alpha
- PMID: 17442832
- PMCID: PMC6672308
- DOI: 10.1523/JNEUROSCI.5368-06.2007
AKAP12 regulates human blood-retinal barrier formation by downregulation of hypoxia-inducible factor-1alpha
Abstract
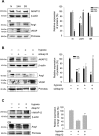
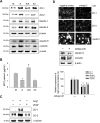
Many diseases of the eye such as retinoblastoma, diabetic retinopathy, and retinopathy of prematurity are associated with blood-retinal barrier (BRB) dysfunction. Identifying the factors that contribute to BRB formation during human eye development and maintenance could provide insights into such diseases. Here we show that A-kinase anchor protein 12 (AKAP12) induces BRB formation by increasing angiopoietin-1 and decreasing vascular endothelial growth factor (VEGF) levels in astrocytes. We reveal that AKAP12 downregulates the level of hypoxia-inducible factor-1alpha (HIF-1alpha) protein by enhancing the interaction of HIF-1alpha with pVHL (von Hippel-Lindau tumor suppressor protein) and PHD2 (prolyl hydroxylase 2). Conditioned media from AKAP12-overexpressing astrocytes induced barriergenesis by upregulating the expression of tight junction proteins in human retina microvascular endothelial cells (HRMECs). Compared with the retina during BRB maturation, AKAP12 expression in retinoblastoma patient tissue was markedly reduced whereas that of VEGF was increased. These findings suggest that AKAP12 may induce BRB formation through antiangiogenesis and barriergenesis in the developing human eye and that defects in this mechanism can lead to a loss of tight junction proteins and contribute to the development of retinal pathologies such as retinoblastoma.
Figures








References
-
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. - PubMed
-
- Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279:38458–38465. - PubMed
-
- Brown RC, Davis TP. Hypoxia/aglycemia alters expression of occludin and actin in brain endothelial cells. Biochem Biophys Res Commun. 2005;327:1114–1123. - PubMed
-
- Brown RC, Mark KS, Egleton RD, Davis TP. Protection against hypoxia-induced blood-brain barrier disruption: changes in intracellular calcium. Am J Physiol Cell Physiol. 2004;286:C1045–1052. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
