MTOC reorientation occurs during FcgammaR-mediated phagocytosis in macrophages
- PMID: 17442887
- PMCID: PMC1924806
- DOI: 10.1091/mbc.e06-12-1128
MTOC reorientation occurs during FcgammaR-mediated phagocytosis in macrophages
Abstract
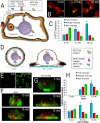
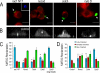
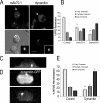
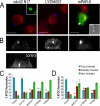
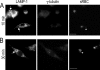
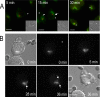
Cell polarization is essential for targeting signaling elements and organelles to active plasma membrane regions. In a few specialized cell types, cell polarity is enhanced by reorientation of the MTOC and associated organelles toward dynamic membrane sites. Phagocytosis is a highly polarized process whereby particles >0.5 microm are internalized at stimulated regions on the cell surface of macrophages. Here we provide detailed evidence that the MTOC reorients toward the site of particle internalization during phagocytosis. We visualized MTOC proximity to IgG-sRBCs in fixed RAW264.7 cells, during live cell imaging using fluorescent chimeras to label the MTOC and using frustrated phagocytosis assays. MTOC reorientation in macrophages is initiated by FcgammaR ligation and is complete within 1 h. Polarization of the MTOC toward the phagosome requires the MT cytoskeleton and dynein motor activity. cdc42, PI3K, and mPAR-6 are all important signaling molecules for MTOC reorientation during phagocytosis. MTOC reorientation was not essential for particle internalization or phagolysosome formation. However Golgi reorientation in concert with MTOC reorientation during phagocytosis implicates MTOC reorientation in antigen processing events in macrophages.
Figures


Similar articles
-
Cdc42, dynein, and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization.Curr Biol. 2001 Oct 2;11(19):1536-41. doi: 10.1016/s0960-9822(01)00475-4. Curr Biol. 2001. PMID: 11591323
-
The Rap1-cofilin-1 pathway coordinates actin reorganization and MTOC polarization at the B cell immune synapse.J Cell Sci. 2017 Mar 15;130(6):1094-1109. doi: 10.1242/jcs.191858. Epub 2017 Feb 6. J Cell Sci. 2017. PMID: 28167682
-
Microtubule involvement in NIH 3T3 Golgi and MTOC polarity establishment.J Cell Sci. 2003 Feb 15;116(Pt 4):743-56. doi: 10.1242/jcs.00288. J Cell Sci. 2003. PMID: 12538774
-
Golgi as an MTOC: making microtubules for its own good.Histochem Cell Biol. 2013 Sep;140(3):361-7. doi: 10.1007/s00418-013-1119-4. Epub 2013 Jul 3. Histochem Cell Biol. 2013. PMID: 23821162 Free PMC article. Review.
-
Signaling and membrane dynamics during phagocytosis: many roads lead to the phagos(R)ome.Curr Opin Cell Biol. 2004 Aug;16(4):422-8. doi: 10.1016/j.ceb.2004.06.006. Curr Opin Cell Biol. 2004. PMID: 15261675 Review.
Cited by
-
Human TRIM gene expression in response to interferons.PLoS One. 2009;4(3):e4894. doi: 10.1371/journal.pone.0004894. Epub 2009 Mar 17. PLoS One. 2009. PMID: 19290053 Free PMC article.
-
Cellular localization of NLRP3 inflammasome.Protein Cell. 2013 Jun;4(6):425-31. doi: 10.1007/s13238-013-2113-2. Epub 2013 Apr 23. Protein Cell. 2013. PMID: 23609011 Free PMC article.
-
The microglial activation state regulates migration and roles of matrix-dissolving enzymes for invasion.J Neuroinflammation. 2013 Jun 21;10:75. doi: 10.1186/1742-2094-10-75. J Neuroinflammation. 2013. PMID: 23786632 Free PMC article.
-
Modulation of Immune Responses by Particle Size and Shape.Front Immunol. 2021 Feb 12;11:607945. doi: 10.3389/fimmu.2020.607945. eCollection 2020. Front Immunol. 2021. PMID: 33679696 Free PMC article. Review.
-
Interactions between Leishmania braziliensis and Macrophages Are Dependent on the Cytoskeleton and Myosin Va.J Parasitol Res. 2012;2012:275436. doi: 10.1155/2012/275436. Epub 2012 Jun 27. J Parasitol Res. 2012. PMID: 22792440 Free PMC article.
References
-
- Ackerman A. L., Kyritsis C., Tampe R., Cresswell P. Access of soluble antigens to the endoplasmic reticulum can explain cross-presentation by dendritic cells. Nat. Immunol. 2005;6:107–113. - PubMed
-
- Athlin L., Domellof L., Norberg B. O. The phagocytosis of yeast cells by monocytes: effects of colchicine, beta-lumicolchicine, and gamma-lumicolchicine. Lymphology. 1986;19:130–135. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

