A Mutation in the SH2 domain of STAT2 prolongs tyrosine phosphorylation of STAT1 and promotes type I IFN-induced apoptosis
- PMID: 17442890
- PMCID: PMC1924825
- DOI: 10.1091/mbc.e06-09-0843
A Mutation in the SH2 domain of STAT2 prolongs tyrosine phosphorylation of STAT1 and promotes type I IFN-induced apoptosis
Abstract
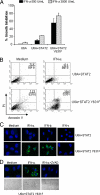
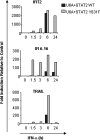
Type I interferons (IFN-alpha/beta) induce apoptosis in certain tumor cell lines but not others. Here we describe a mutation in STAT2 that confers an apoptotic effect in tumor cells in response to type I IFNs. This mutation was introduced in a conserved motif, PYTK, located in the STAT SH2 domain, which is shared by STAT1, STAT2, and STAT3. To test whether the tyrosine in this motif might be phosphorylated and affect signaling, Y631 of STAT2 was mutated to phenylalanine (Y631F). Although it was determined that Y631 was not phosphorylated, the Y631F mutation conferred sustained signaling and induction of IFN-stimulated genes. This prolonged IFN response was associated with sustained tyrosine phosphorylation of STAT1 and STAT2 and their mutual association as heterodimers, which resulted from resistance to dephosphorylation by the nuclear tyrosine phosphatase TcPTP. Finally, cells bearing the Y631F mutation in STAT2 underwent apoptosis after IFN-alpha stimulation compared with wild-type STAT2. Therefore, this mutation reveals that a prolonged response to IFN-alpha could account for one difference between tumor cell lines that undergo IFN-alpha-induced apoptosis compared with those that display an antiproliferative response but do not die.
Figures



Similar articles
-
Heartland virus antagonizes type I and III interferon antiviral signaling by inhibiting phosphorylation and nuclear translocation of STAT2 and STAT1.J Biol Chem. 2019 Jun 14;294(24):9503-9517. doi: 10.1074/jbc.RA118.006563. Epub 2019 Apr 30. J Biol Chem. 2019. PMID: 31040183 Free PMC article.
-
Identification of a novel conserved motif in the STAT family that is required for tyrosine phosphorylation.J Biol Chem. 2004 Mar 26;279(13):12379-85. doi: 10.1074/jbc.M310787200. Epub 2004 Jan 13. J Biol Chem. 2004. PMID: 14722125
-
Functional subdomains of STAT2 required for preassociation with the alpha interferon receptor and for signaling.Mol Cell Biol. 1997 Apr;17(4):2048-56. doi: 10.1128/MCB.17.4.2048. Mol Cell Biol. 1997. PMID: 9121453 Free PMC article.
-
A Positive Feedback Amplifier Circuit That Regulates Interferon (IFN)-Stimulated Gene Expression and Controls Type I and Type II IFN Responses.Front Immunol. 2018 May 28;9:1135. doi: 10.3389/fimmu.2018.01135. eCollection 2018. Front Immunol. 2018. PMID: 29892288 Free PMC article. Review.
-
The role of signal transducer and activator of transcription-2 in the interferon response.J Interferon Cytokine Res. 2012 Mar;32(3):103-10. doi: 10.1089/jir.2011.0099. Epub 2012 Jan 26. J Interferon Cytokine Res. 2012. PMID: 22280068 Free PMC article. Review.
Cited by
-
STAT2: A shape-shifting anti-viral super STAT.JAKSTAT. 2013 Jan 1;2(1):e23633. doi: 10.4161/jkst.23633. JAKSTAT. 2013. PMID: 24058798 Free PMC article. Review.
-
Interferons as inducers of apoptosis in malignant cells.J Interferon Cytokine Res. 2013 Apr;33(4):162-70. doi: 10.1089/jir.2012.0110. J Interferon Cytokine Res. 2013. PMID: 23570382 Free PMC article. Review.
-
Identification of STAT2 serine 287 as a novel regulatory phosphorylation site in type I interferon-induced cellular responses.J Biol Chem. 2013 Jan 4;288(1):747-58. doi: 10.1074/jbc.M112.402529. Epub 2012 Nov 8. J Biol Chem. 2013. PMID: 23139419 Free PMC article.
-
Immunotherapeutic implications of negative regulation by protein tyrosine phosphatases in T cells: the emerging cases of PTP1B and TCPTP.Front Med (Lausanne). 2024 Apr 19;11:1364778. doi: 10.3389/fmed.2024.1364778. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38707187 Free PMC article. Review.
-
Responses to Cytokines and Interferons that Depend upon JAKs and STATs.Cold Spring Harb Perspect Biol. 2018 Jan 2;10(1):a028555. doi: 10.1101/cshperspect.a028555. Cold Spring Harb Perspect Biol. 2018. PMID: 28620095 Free PMC article. Review.
References
-
- Banninger G., Reich N. C. STAT2 nuclear trafficking. J. Biol. Chem. 2004;279:39199–39206. - PubMed
-
- Bleumer I., Oosterwijk E., De Mulder P., Mulders P. F. Immunotherapy for renal cell carcinoma. Eur. Urol. 2003;44:65–75. - PubMed
-
- Brierley M. M., Fish E. N. Functional relevance of the conserved DNA-binding domain of STAT2. J. Biol. Chem. 2005;280:13029–13036. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

