DC immunotherapy is highly effective for the inhibition of tumor metastasis or recurrence, although it is not efficient for the eradication of established solid tumors
- PMID: 17443323
- PMCID: PMC11029899
- DOI: 10.1007/s00262-007-0325-0
DC immunotherapy is highly effective for the inhibition of tumor metastasis or recurrence, although it is not efficient for the eradication of established solid tumors
Abstract
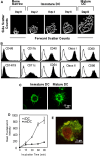
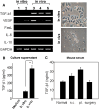
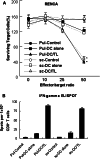
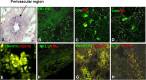
Dendritic cell (DC)-based immunotherapy has not been as effective as expected in most solid tumors even in the murine model, particularly in renal cell carcinoma (RCC). Our investigation was initiated to identify what causes the limitations of DC-based immunotherapy in solid RCC. We have investigated immunosuppressive factors from tumors and their effects on DC migration, as well as cytotoxic T lymphocyte (CTL) response and lymphocyte infiltration into the tumor mass upon vaccination with mouse renal adenocarcinoma (Renca) cell lysate-pulsed bone marrow (Bm)-derived DC in tumor-bearing mice. We also investigated pulmonary metastasis- and tumor recurrence-inhibitory effects of DC-vaccination in the solid tumor-bearing mice. In these experiments, we found that the limitations of DC-based immunotherapy to solid RCC likely result from tumor-mediated TGF-beta hindrance of immune attack rather than insufficient immune induction by DC therapy. In fact, the CTL response induced by DC therapy was quite sufficient and functional for the inhibition of tumor recurrence after surgery or of tumor metastasis induced by additional tumor-challenge to the tumor-bearing mice. Taken together, our present results obtained in mouse model suggest the potential of DC immunotherapy in tumor patients for hindering or blocking disease progression by inhibition of tumor metastasis and/or tumor recurrence after surgery.
Figures



References
-
- Ahn JH, Lee Y, Jeon C, Lee SJ, Lee BH, Choi KD, Bae YS. Identification of the genes differentially expressed in human dendritic cell subsets by cDNA subtraction and microarray analysis. Blood. 2002;100:1742–1754. - PubMed
-
- Arlen PM, Gulley JL. Therapeutic vaccines for prostate cancer: a review of clinical data. Curr Opin Investig Drugs. 2005;6:592–596. - PubMed
-
- Asselin-Paturel C, Echchakir H, Carayol G, Gay F, Opolon P, Grunenwald D, Chouaib S, Mami-Chouaib F. Quantitative analysis of Th1, Th2 and TGF-beta1 cytokine expression in tumor, TIL and PBL of non-small cell lung cancer patients. Int J Cancer. 1998;77:7–12. doi: 10.1002/(SICI)1097-0215(19980703)77:1<7::AID-IJC2>3.0.CO;2-Y. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

