Nitric oxide for preventing pre-eclampsia and its complications
- PMID: 17443623
- PMCID: PMC8985412
- DOI: 10.1002/14651858.CD006490
Nitric oxide for preventing pre-eclampsia and its complications
Abstract
Background: Pre-eclampsia, a multisystem disorder of pregnancy characterised by high blood pressure and protein in the urine, is associated with endothelial dysfunction. Nitric oxide mediates many functions of the endothelium, including vasodilatation and inhibition of platelet aggregation. Pre-eclampsia may be associated with nitric oxide deficiency, but the evidence to support this suggestion is contradictory. Nevertheless, it has been hypothesised that agents which increase nitric oxide may prevent pre-eclampsia.
Objectives: To assess the effectiveness and safety of nitric oxide donors and precursors for preventing pre-eclampsia and its complications.
Search strategy: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (November 2006), CENTRAL (The Cochrane Library 2006, Issue 3), and EMBASE (2002 to December 2004).
Selection criteria: Studies were included if they were randomised trials evaluating nitric oxide donors or precursors for preventing pre-eclampsia and its complications.
Data collection and analysis: Both review authors independently assessed studies for inclusion. Data were extracted and double checked for accuracy.
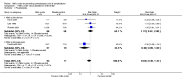
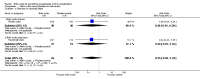
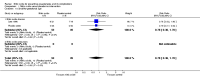
Main results: Six trials (310 women) were included. Four were of good quality and two were of uncertain quality. Four trials (170 women) compared nitric oxide donors (glyceryl trinitrate) or precursors (L-arginine) with either placebo or no intervention. There are insufficient data for reliable conclusions about the effects on pre-eclampsia (four trials, 170 women; relative risk (RR) 0.83, 95% confidence interval (CI) 0.49 to 1.41) or its complications. One trial (36 women) compared a nitric oxide donor with nifedipine, and another (76 women) compared it with antiplatelet agents. Both were too small for reliable conclusions about possible differential effects. Glyceryl trinitrate was associated with an increased risk of headache (two trials, 56 women; RR 6.85, 95% CI 1.42 to 33.04), and of stopping treatment (two trials, 56 women; RR 4.02, 95% CI 1.15 to 14.09) compared to placebo. However, the increase for both outcomes was due to an extreme result in one small trial (7/7 versus 0/9 for both outcomes).
Authors' conclusions: There is insufficient evidence to draw reliable conclusions about whether nitric oxide donors and precursors prevent pre-eclampsia or its complications.
Conflict of interest statement
None known.
Figures
























References
References to studies included in this review
Davis 2001 {published and unpublished data}
-
- Davis G, Brown M. Glyceryl trinitrate patches are unsuitable in hypertensive pregnancy. Australian and New Zealand Journal of Obstetrics and Gynaecology 2001;41(4):474. - PubMed
Facchinetti 2002 {published and unpublished data}
-
- Facchinetti F, Piccinini F, Pizzi C, Bukowski R, Volpe A, Saade G. Effect of arginine supplementation in patients with gestational hypertension. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S213.
Lees 1998 {published data only}
-
- Lees C, Valensise H, Black R, Harrington K, Byiers S, Romanini C, et al. The efficacy and fetal‐maternal cardiovascular effects of transdermal glyceryl trinitrate in the prophylaxis of pre‐eclampsia and its complications: a randomized double‐blind placebo‐controlled trial. Ultrasound in Obstetrics & Gynecology 1998;12(5):334‐8. [CN‐00157005] - PubMed
Neri 1999 {published and unpublished data}
-
- Neri I, Valensise H, Facchinetti F, Menghini S, Romanini C, Volpe A. 24‐hour ambulatory blood pressure monitoring: a comparison between transdermal glyceryl‐trinitrate and oral nifedipine. Hypertension in Pregnancy 1999;18(1):107‐13. [CN‐00233632] - PubMed
Picciolo 2000 {published data only}
-
- Picciolo C, Roncaglia N, Neri I, Pasta F, Arreghini A, Facchinetti F. Nitric oxide in the prevention of pre‐eclampsia. Prenatal and Neonatal Medicine 2000;5(4):212‐5. [CN‐00399568]
Zozulia 1997 {published data only}
-
- Zozulia O, Rogov VA, Piatakova NV, Tareeva IE. Nitric oxide: its role in the development of pregnancy complications and in their prevention in women with hypertension and chronic glomerulonephritis. Terapevticheskii Arkhiv 1997;69(6):17‐20. - PubMed
References to studies excluded from this review
Amit 1998 {published data only}
-
- Amit A, Thaler I, Paz Y, Itskovitz‐Eldor J. The effect of a nitric oxide donor on Doppler flow velocity waveforms in the uterine artery during the first trimester of pregnancy. Ultrasound in Obstetrics & Gynecology 1998;11(2):94‐8. [CN‐00304196] - PubMed
Decano 2000 {published data only}
-
- Decano MB, Cabrera LT. The effects of transdermal nitroglycerin (nitrol patch) on the uterine and umbilical artery blood flow in preeclampsia: a randomized double blind placebo controlled study [abstract]. XVI FIGO World Congress of Obstetrics & Gynecology (Book 1); 2000 Sept 3‐8; Washington DC, USA. 2000:26. [CN‐00355035]
Hladunewich 2006 {published data only}
-
- Hladunewich MA, Derby GC, Lafayette RA, Blouch KL, Druzin ML, Myers BD. Effect of L‐arginine therapy on the glomerular injury of preeclampsia. Obstetrics & Gynecology 2006;107(4):886‐95. - PubMed
Ledingham 1999 {published data only}
-
- Ledingham MA, Denison FC, Kelly RW, Young A, Normal JE. Nitric oxide donors stimulate prostaglandin F2alpha and inhibit thromboxane B2 production in the human cervix during first trimester of pregnancy. Molecular Human Reproduction 1999;5(10):973‐82. - PubMed
Madhubala 2006 {published data only}
-
- Madhubala M. Use of L‐arginine in oligohydramnios. 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6‐9; Cochin, Kerala State, India. 2006:42.
Makino 1997 {published data only}
-
- Makino Y, Izumi H, Makino I, Shirakawa K. The effect of nitric oxide on uterine and umbilical artery flow velocity waveform in pre‐eclampsia. European Journal of Obstetrics & Gynecology and Reproductive Biology 1997;73(2):139‐43. [CN‐00141718] - PubMed
Neri 2000b {published data only}
-
- Neri I, Ternelli G, Facchinetti F, Volpe A. Effectiveness of oral nifedipine and transdermal glyceryltrinitrate on 24‐hour blood pressure values in pregnancy‐induced hypertension. Hypertension in Pregnancy 2000;19(Suppl 1):O65.
Neri 2004a {published data only}
-
- Neri I, Blasi I, Facchinetti F. Effects of acute L‐arginine infusion on non‐stress test in hypertensive pregnant women. Journal of Maternal‐Fetal & Neonatal Medicine 2004;16:23‐6. - PubMed
Neri 2004b {published data only}
-
- Neri I, Jasonni VM, Gori G, Blasi I, Facchinetti F. Intravenous l‐arginine is a new anti‐hypertensive agent for pregnant women. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S42.
Reyna‐Villasmil 2001 {published data only}
-
- Reyna‐Villasmil E, Prieto‐Franchi M, Guerra‐Velazquez M, Torres‐Montilla M. Effect of transdermal nitroglycerin on umbilical artery blood flow in preeclampsia. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 2):486. [CN‐00363873]
Ruggenenti 2006 {published data only}
-
- Ruggenenti P. L‐Arginine in pre‐eclampsia. www.clinicaltrials.gov (accessed 21 March 2006).
Rytewski 2005 {published data only}
-
- Rytlewski K, Olszanecki R, Korbut R, Zdebski Z. Effects of prolonged oral supplementation with L‐arginine on blood pressure and nitric oxide synthesis in pre‐eclampsia. European Journal of Clinical Investigation 2005;35:32‐7. - PubMed
Rytlewski 2006 {published data only}
-
- Rytlewski K, Olszanecki R, Lauterbach R, Grzyb A, Basta A. Effects of oral l‐arginine on the foetal condition and neonatal outcome in preeclampsia: a preliminary report. Basic & Clinical Pharmacology & Toxicology 2006;99:146‐52. - PubMed
Scarpellini 2001 {published data only}
-
- Scarpellini F, Sbracia M, Lecchini S, Bolis P. Nitric oxide donor treatment reduces apoptosis in placental vessels of pregnancy induced hypertension. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S177. [CN‐00387652]
Staff 2004 {published data only}
-
- Staff AC, Berge L, Haugen G, Lorentzen B, Mikkelsen B, Henriksen T. Dietary supplementation with L‐arginine or placebo in women with pre‐eclampsia. Acta Obstetricia et Gynecologica Scandinavica 2004;83(1):103‐7. [CN‐00459625] - PubMed
Thaler 1999 {published data only}
-
- Thaler I, Amit A, Kamil D, Itskovitz‐Eldor J. The effect of isosorbide dinitrate on placental blood flow and maternal blood pressure in women with pregnancy induced hypertension. American Journal of Hypertension 1999;12(4 Pt 1):341‐7. [CN‐00162808] - PubMed
Valensise 2005 {published data only}
-
- Valensise H, Vasapollo B, Novelli GP, Altomare F, Arduini D. Nitric oxide donors and fluid therapy increase fetal growth in gestational hypertension. Ultrasound in Obstetrics & Gynecology 2005;26:440.
References to studies awaiting assessment
Di Iorio 2002 {published data only}
-
- Iorio R, Marinoni E, Gazzolo D, Letizia C, Netta T, Cosmi EV. Maternal nitric oxide supplementation increases adrenomedullin concentrations in growth retarded fetuses. Gynecological Endocrinology 2002;16(3):187‐92. - PubMed
El‐Hamedi 2001 {published data only}
-
- El‐Hamedi A, Shillito TJA, Simpson NAB, Walker JJ. A prospective randomised controlled trial of nitric oxide donors in at risk pregnancy [abstract]. Journal of Obstetrics and Gynaecology 2001;21 Suppl 1:S22.
Facchinetti 2007 {published data only}
-
- Facchinetti F, Saade GR, Neri I, Pizzi C, Longo M, Volpe A. L‐arginine supplementation in patients with gestational hypertension: a pilot study. Hypertension in Pregnancy 2007;26(1):121‐30. - PubMed
Facchinetti 2008 {published data only}
-
- Facchinetti F. Effects of oral L‐arginine on chronic hypertension in pregnancy. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 February 2008).
Hubel 2009 {published data only}
-
- Hubel CA. Oral L‐arginine and ADMA in pregnancy. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 4 January 2009) 2009.
Lopez‐Molina 2008 {published data only}
-
- Lopez‐Molina K, Gonzalez‐Altamirano JC, Valdivia‐Silva JE. Early L‐arginine therapy improves notably the fetal growth in preeclamptic women. A randomized controlled trial. 55th Annual Meeting of the Society of Gynecologic Investigation; 2008 March 26‐29; San Diego, USA 2008:Abstract no: 801.
Neri 2006 {published data only}
-
- Neri I, Jasonni VM, Gori GF, Blasi I, Facchinetti F. Effect of L‐arginine on blood pressure in pregnancy‐induced hypertension: a randomized placebo‐controlled trial. Journal of Maternal‐Fetal and Neonatal Medicine 2006;19(5):277‐81. - PubMed
Noris 2008 {published data only}
-
- Noris M, Fratelli N, Rampello S, Gherardi G, Platto C, Todeschini M, et al. A double‐blind, randomized, pilot study on L‐arginine oral supplementation in pre‐eclamptic pregnancies. Hypertension in Pregnancy 2008;27(4):505.
Samangaya 2009 {published data only}
-
- Samangaya RA, Mires G, Shennan A, Skillern L, Howe D, McLeod A, et al. A randomised, double‐blinded, placebo‐controlled trial of the phosphodiesterase type 5 inhibitor sildenafil in the treatment of preeclampsia. Hypertension in Pregnancy 2009;28:369‐82. - PubMed
Smith 2008 {published data only}
-
- Smith G. Use of a nitric oxide (ISMN) for the prevention and management of pre‐eclampsia (pilot study). Current Controlled Trials (www.controlled‐trials.com/) (accessed 20 February 2008) 2008.
Winer 2007 {published data only}
-
- Winer N, Branger B, Azria E, ROze JC, Descamps P, Philippe HJ, et al. Treatment of severe vascular fetal intrauterine growth restriction with L‐arginine: a multicenter prospective double‐blind randomized placebo‐controlled study [abstract]. Ultrasound in Obstetrics and Gynecology 2007;30:381.
Xiao 2005 {published data only}
-
- Xiao XM, Li LP. L‐arginine treatment for asymmetric fetal growth restriction. International Journal of Gynecology & Obstetrics 2005;88(1):15‐8. - PubMed
Zhang 2007 {published data only}
-
- Zhang N, Xiong AH, Xiao X, Li LP. Effect and mechanism of L‐arginine therapy for fetal growth retardation due to pregnancy‐induced hypertension. Nan Fang Yi Ke Da Xue Xue Bao//Journal of Southern Medical University 2007;27(2):198‐200. - PubMed
Additional references
Benedetto 2000
-
- Benedetto C, Marozio L, Neri I, Giarola M, Volpe A, Facchinetti F. Increased L‐citrulline/L‐arginine plasma ratio in severe preeclampsia. Obstetrics & Gynecology 2000;96(3):395‐9. - PubMed
Cockell 1997
-
- Cockell AP, Poston L. Flow‐mediated vasodilatation is enhanced in normal pregnancy but reduced in preeclampsia. Hypertension 1997;30(2):247‐51. - PubMed
Conrad 1999
-
- Conrad KP, Kerchner LJ, Mosher MD. Plasma and 24‐h NOx and cGMP during normal pregnancy and preeclampsia in women on a reduced NOx diet. American Journal of Physiology 1999;2777(1):F48‐F57. - PubMed
Davidge 1996
-
- Davidge ST, Stranko CP, Roberts JM. Urine but not plasma nitric oxide metabolites are decreased in women with preeclampsia. American Journal of Obstetrics and Gynecology 1996;174(3):1008‐13. - PubMed
De Caterina 1995
-
- Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA Jr, et al. Nitric oxide decreases cytokine‐induced endothelial activation. Nitric oxide selectively reduces expression of adhesion molecules and proinflammatory cytokines. Journal of Clinical Investigation 1995;96(1):60‐8. - PMC - PubMed
Delacretaz 1995
-
- Delacretaz E, DeQuay N, Waeber B, Vial Y, Schulz PE, Burnier M, et al. Differential nitric oxide synthase activity in human platelets during normal pregnancy and pre‐eclampsia. Clinical Sciences 1995;88:607‐10. - PubMed
DH 2002
-
- Department of Health, Scottish Executive Health Department and Department of Health, Social Services, Public Safety. Northern Ireland. Why mothers die. The sixth report on confidential enquiries into maternal deaths in the United Kingdom 2000‐2002. London: RCOG Press, 2002.
Di Iorio 1998
-
- Ioro R, Marinoni E, Emiliani S, Villaccio B, Ermelando VC. Nitric oxide in preeclampsia: lack of evidence for decreased production. European Journal of Obstetrics & Gynecology and Reproductive Biology 1998;76(1):65‐70. - PubMed
Fickling 1993
-
- Fickling SA, Williams D, Vallance P, Nussey SS, Whitley GStJ. Plasma concentrations of endogenous inhibitor of nitric oxide synthesis in normal pregnancy and pre‐eclampsia. Lancet 1993;342:242‐3. - PubMed
Generic Protocol 2005
-
- Meher S, Duley L, Prevention of Pre‐eclampsia Cochrane Review Authors. Interventions for preventing pre‐eclampsia and its consequences: generic protocol. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD005301] - DOI
Grunewald 1995
-
- Grunewald C, Kublickas M, Carlstrom K, Lunell N, Nisell H. Effects of nitroglycerin on the uterine and umbilical circulation in severe preeclampsia. Obstetrics & Gynecology 1995;86:600‐4. - PubMed
Higgins 2005
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.4 [updated March 2005. In: The Cochrane Library, Issue 2, 2005. Chichester, UK: John Wiley & Sons, Ltd.
Hubel 1997
-
- Hubel CA, Kagan VE, Kisin ER, McLaughlin MK, Roberts JM. Increased ascorbate radical formation and ascorbate depletion in plasma from women with preeclampsia: implications for oxidative stress. Free Radical Biology and Medicine 1997;23:597‐609. - PubMed
Ignarro 1987
Kubes 1991
Lees 1996
-
- Lees C, Langford E, Brown AS, Belder A, Pickles A, Martin JF. The effects of S‐nitrosoglutathione on platelet activation, hypertension, and uterine and fetal Doppler in severe preeclampsia. Obstetrics & Gynecology 1996;88:14‐9. - PubMed
Lowe 2000
-
- Lowe DT. Nitric oxide dysfunction in the pathophysiology of preeclampsia. Nitric Oxide 2000;4(4):441‐58. - PubMed
Lyall 1995
-
- Lyall F, Young A, Greer IA. Nitric oxide concentrations are increased in the fetoplacental circulation in preeclampsia. American Journal of Obstetrics and Gynecology 1995;173(1):714‐8. - PubMed
Molnar 1994
-
- Molnar M, Suto T, Toth T, Hertelendy F. Prolonged blockade of nitric oxide synthesis in gravid rats produces sustained hypertension, proteinuria, thrombocytopenia, and intrauterine growth retardation. American Journal of Obstetrics and Gynecology 1994;170(1):1458‐66. - PubMed
Moncada 1991
-
- Moncada S, Palmer RMJ, Higgs EA. Nitric oxide. Pharmacological Reviews 1991;43(2):109‐42. - PubMed
Morris 1995
-
- Morris NH, Sooranna SR, Learmont JG, Poston L, Ramsey B, Peason JD, et al. Nitric oxide synthase activities in placental tissue from normotensive, preeclamptic, and growth retarded fetuses. British Journal of Obstetrics and Gynaecology 1995;102:711‐4. - PubMed
Myatt 1992
-
- Myatt L, Brewer AS, Langdon G, Brockman DE. Attenuation of the vasoconstrictor effects of thromboxane and endothelin by nitric oxide in the human fetal‐placental circulation. American Journal of Obstetrics and Gynecology 1992;166:224‐30. - PubMed
Neri 2000a
-
- Neri I, Piccinini F, Marietta M, Facchinetti F, Volpe A. Platelet responsiveness to L‐arginine in hypertensive disorders of pregnancy. Hypertension in Pregnancy 2000;19(3):323‐30. - PubMed
NHBPEP2000
-
- Gifford RW Jr, August PA, Cunningham G, Green LA, Lindhemier MD, McNellis D, et al. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. American Journal of Obstetrics and Gynecology 2000;183(1):S1‐S22. - PubMed
Noris 2004
-
- Noris M, Todeschini M, Cassis P, Pasta F, Cappellini A, Bonazzola S, et al. L‐arginine depletion in preeclampsia orients nitric oxide synthase toward oxidant species. Hypertension 2004;43(3):614‐22. - PubMed
Norris 1999
-
- Norris L, Higgins JR, Darling MR, Walshe JJ, Bonnar J. Nitric oxide in the uteroplacental, fetoplacental, and peripheral circulation in preeclampsia. Obstetrics & Gynecology 1999;93(6):958‐63. - PubMed
Palmer 1987
-
- Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium‐derived relaxing factor. Nature 1987;327:524‐6. - PubMed
Pinto 1991
-
- Pinto A, Sorrentino R, Sorrentino P, Guerritore T, Miranda L, Biondi A, et al. Endothelial‐derived relaxing factor released by endothelial cells of human umbilical vessels and its impairment in pregnancy‐induced hypertension. American Journal of Obstetrics and Gynecology 1991;164:507‐13. - PubMed
Poston 1998
-
- Poston L. Nitrovasodilators ‐ will they be useful in lowering uterine artery resistance in pre‐eclampsia and intrauterine growth restriction?. Ultrasound in Obstetrics & Gynecology 1998;11:92‐3. - PubMed
Radomski 1987
-
- Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 1987;2(8567):1057‐8. - PubMed
Ranta 1999
-
- Ranta V, Viinikka L, Halmesmaki E, Ylikorkala O. Nitric oxide production with preeclampsia. Obstetrics & Gynecology 1999;93(3):442‐5. - PubMed
RevMan 2003 [Computer program]
-
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Oxford, England: The Cochrane Collaboration, 2003.
Seligman 1994
-
- Seligman SP, Buyon JP, Clancy RM, Young BK, Abramson SB. The role of nitric oxide in the pathogenesis of preeclampsia. American Journal of Obstetrics and Gynecology 1994;171:944‐8. - PubMed
Shaamash 2001
-
- Shaamash AH, Elsonosy ED, Zakhari MM, Radwan SH, El‐Dien HM. Placental nitric oxide synthase (NOS) activity and nitric oxide (NO) production in normal pregnancy, pre‐eclampsia, and eclampsia. International Journal of Gynecology & Obstetrics 2001;72(2):127‐33. - PubMed
Silver 1996
-
- Silver RK, Kupferminc MJ, Russell TL, Adler L, Mullen TA, Caplan MS. Evaluation of nitric oxide as a mediator of severe preeclampsia. American Journal of Obstetrics and Gynecology 1996;175(4):1013‐7. - PubMed
Sladek 1997
-
- Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. American Journal of Physiology 1997;272:R441‐63. - PubMed
WHO 1988
-
- World Health Organization International Collaborative Study of Hypertensive Disorders in Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. American Journal of Obstetrics and Gynecology 1988;158:80‐3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

