The adipose cell lineage is not intrinsically insulin resistant in polycystic ovary syndrome
- PMID: 17445549
- PMCID: PMC2427369
- DOI: 10.1016/j.metabol.2006.12.021
The adipose cell lineage is not intrinsically insulin resistant in polycystic ovary syndrome
Abstract
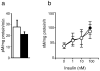
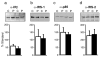
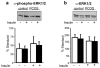
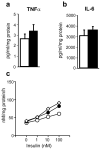
Selective resistance to the effects of insulin on glucose metabolism in skeletal muscle and adipose tissue is a key feature of polycystic ovary syndrome (PCOS). The pathogenesis of insulin resistance in skeletal muscle in PCOS involves interaction of in vivo environmental factors with intrinsic defects in insulin signaling. We aimed to determine whether (1) intrinsic defects in insulin action/signaling and cytokine secretion were present in adipose cells in PCOS and (2) insulin resistance can be induced in control adipose cells by culture in medium conditioned by insulin-resistant PCOS fibroblasts. Subcutaneous abdominal preadipocytes from obese women with PCOS (n = 7) and age- and body mass index-matched controls (n = 5) were cultured for several generations in vitro. Basal and insulin-stimulated glycogen synthesis and basal glucose transport did not differ in the preadipocytes from women with PCOS and controls. Abundance of insulin receptor (IR) beta subunit, insulin receptor substrate (IRS) 1 and 2, p85 subunit of phosphatidylinositol 3-kinase, and extracellular signal-regulated kinase (ERK)1/2 activation did not differ. Secretion of tumor necrosis factor alpha and interleukin 6 did not differ. Insulin action on glycogen synthesis in control preadipocytes was not altered by coculture with or growth in media conditioned by PCOS skin fibroblasts with constitutive serine phosphorylation of IRbeta subunit (IR ser+), indicating that IR ser+ cells do not secrete an insulin resistance-inducing factor. We conclude that in contrast to skeletal muscle and skin fibroblasts, there is no evidence for intrinsic defects in insulin signaling in the PCOS adipose cell lineage, indicating that insulin resistance in these cells is likely due to factors in the in vivo environment.
Figures
Similar articles
-
Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling.Am J Physiol Endocrinol Metab. 2005 May;288(5):E1047-54. doi: 10.1152/ajpendo.00361.2004. Epub 2004 Dec 21. Am J Physiol Endocrinol Metab. 2005. PMID: 15613682
-
Defects in insulin receptor signaling in vivo in the polycystic ovary syndrome (PCOS).Am J Physiol Endocrinol Metab. 2001 Aug;281(2):E392-9. doi: 10.1152/ajpendo.2001.281.2.E392. Am J Physiol Endocrinol Metab. 2001. PMID: 11440917 Clinical Trial.
-
Enhanced mitogenic signaling in skeletal muscle of women with polycystic ovary syndrome.Diabetes. 2006 Mar;55(3):751-9. doi: 10.2337/diabetes.55.03.06.db05-0453. Diabetes. 2006. PMID: 16505239
-
Metabolism and insulin signaling in common metabolic disorders and inherited insulin resistance.Dan Med J. 2014 Jul;61(7):B4890. Dan Med J. 2014. PMID: 25123125 Review.
-
Insulin resistance in polycystic ovary syndrome: progress and paradoxes.Recent Prog Horm Res. 2001;56:295-308. doi: 10.1210/rp.56.1.295. Recent Prog Horm Res. 2001. PMID: 11237218 Review.
Cited by
-
Sheep models of polycystic ovary syndrome phenotype.Mol Cell Endocrinol. 2013 Jul 5;373(1-2):8-20. doi: 10.1016/j.mce.2012.10.005. Epub 2012 Oct 16. Mol Cell Endocrinol. 2013. PMID: 23084976 Free PMC article. Review.
-
Identification of differentially expressed microRNAs in the ovary of polycystic ovary syndrome with hyperandrogenism and insulin resistance.Chin Med J (Engl). 2015 Jan 20;128(2):169-74. doi: 10.4103/0366-6999.149189. Chin Med J (Engl). 2015. PMID: 25591557 Free PMC article.
-
Evidence that obesity and androgens have independent and opposing effects on gonadotropin production from puberty to maturity.Brain Res. 2010 Dec 10;1364:186-97. doi: 10.1016/j.brainres.2010.08.088. Epub 2010 Sep 25. Brain Res. 2010. PMID: 20816944 Free PMC article. Review.
-
Steroidogenic regulatory factor FOS is underexpressed in polycystic ovary syndrome (PCOS) adipose tissue and genetically associated with PCOS susceptibility.J Clin Endocrinol Metab. 2012 Sep;97(9):E1750-7. doi: 10.1210/jc.2011-2153. Epub 2012 Jun 21. J Clin Endocrinol Metab. 2012. PMID: 22723319 Free PMC article.
-
Polycystic ovary syndrome is associated with tissue-specific differences in insulin resistance.J Clin Endocrinol Metab. 2009 Jan;94(1):157-63. doi: 10.1210/jc.2008-1492. Epub 2008 Oct 14. J Clin Endocrinol Metab. 2009. PMID: 18854391 Free PMC article.
References
-
- Dunaif A. Insulin resistance and the polycystic ovary syndrome; mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. - PubMed
-
- Legro RS, Kunselman A, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–169. - PubMed
-
- Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22:141–146. - PubMed
-
- Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–1174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

